The study of the properties of matter forms one of the most essential building blocks of chemistry. It allows scientists and students to understand how substances behave, how they interact, and how they change under various physical and chemical conditions. For a JEE aspirant, this topic connects deeply with numerous other areas such as thermodynamics, kinetics, and stoichiometry. It also serves as the stepping stone for experimental and theoretical chemistry, linking observation with mathematical precision.
Chemistry is an empirical science, meaning that much of what we know is based on careful measurement and observation. Quantifying the properties of matter is therefore central to building scientific models and equations that describe the real world. Whether determining the boiling point of a compound, measuring the density of a solution, or analyzing the rate of a reaction, understanding how to measure matter accurately is critical.
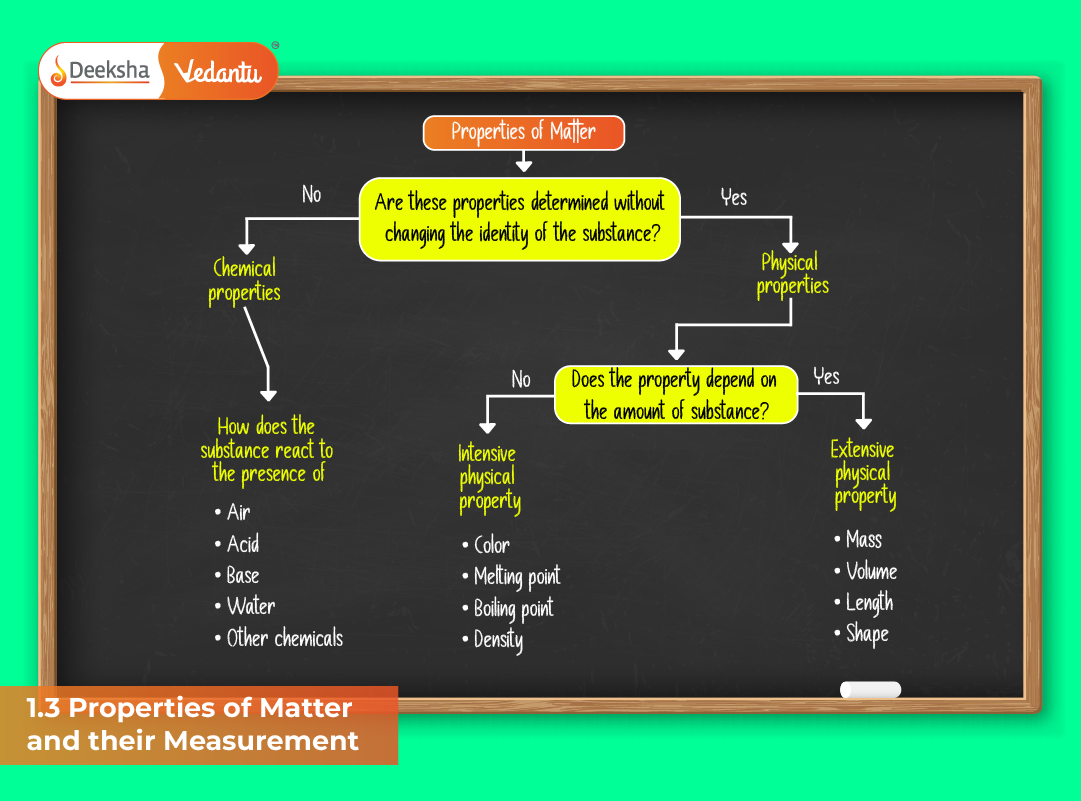
Physical and Chemical Properties
Matter exhibits several measurable or observable features. These are broadly divided into physical and chemical properties.
Physical Properties
Physical properties can be observed without altering the chemical composition of a substance. These include:
- Color, odor, hardness, texture
- Melting and boiling points
- Density and refractive index
- Electrical and thermal conductivity
Example: The melting of ice into water is purely a physical process. The substance remains H₂O before and after melting.
Detailed Explanation: Physical properties arise mainly from intermolecular forces and arrangements. For instance, the high melting point of ionic solids is due to strong electrostatic attraction between ions, while molecular solids have lower melting points due to weak Van der Waals forces.
Real-life Connection: Measuring physical properties like viscosity and surface tension helps industries determine product quality, such as in lubricants or paints.
Chemical Properties
Chemical properties describe how a substance reacts or transforms into one or more new substances. These changes are accompanied by rearrangement of atoms and bonds.
- Reactivity with acids, bases, or oxygen
- Flammability and oxidation states
- Acidity, basicity, or catalytic behavior
Example: The rusting of iron (Fe + O₂ → Fe₂O₃) demonstrates a chemical change resulting in a new compound.
JEE Insight: Many physical chemistry questions hinge on identifying whether a process is physical or chemical. For instance, dissolving NaCl in water is physical (no new substance), while burning methane is chemical.
Sample Problem: Identify whether the following are physical or chemical changes:
- Sublimation of iodine → Physical
- Burning of magnesium ribbon → Chemical
- Dissolving CO₂ in water → Physical
Answer: 1 and 3 are physical changes; 2 is chemical.
Measurement of Physical Properties
Measurement lies at the heart of scientific inquiry. Without accurate measurements, experimental chemistry becomes speculative. Every physical quantity is represented by a numerical value and a unit of measurement.
Formula:
Quantity = Numerical Value × Unit
For example, a sample of gold weighing 2.5 kg implies that its mass is 2.5 times the standard kilogram.
Importance of Measurement
- Enables reproducibility of experiments
- Establishes quantitative relationships (like Boyle’s or Charles’s laws)
- Forms the basis of dimensional analysis
Accuracy vs. Precision:
- Accuracy: Closeness to the actual or true value.
- Precision: Reproducibility among repeated measurements.
Example: A balance reading 49.8 g, 50.0 g, and 50.1 g for a 50 g sample is both accurate and precise. If readings are 48.0 g, 48.1 g, 48.2 g, they are precise but not accurate.
Common Errors in Measurement:
- Systematic Errors: Due to faulty instruments or calibration.
- Random Errors: Due to unpredictable variations.
- Gross Errors: Due to human mistakes like incorrect reading.
JEE Focus: Questions often test understanding of significant figures, rounding off, and propagation of errors.
The International System of Units (SI)
To ensure uniformity across global experiments, the SI system was adopted as the international standard for measurement.
SI Base Units
| Physical Quantity | Symbol | SI Unit | Symbol |
| Length | l | metre | m |
| Mass | m | kilogram | kg |
| Time | t | second | s |
| Electric current | I | ampere | A |
| Temperature | T | kelvin | K |
| Amount of substance | n | mole | mol |
| Luminous intensity | Iv | candela | cd |
Derived Units
These are obtained by combining base units through mathematical relations:
- Area = m²
- Velocity = m/s
- Force = newton (N = kg·m/s²)
- Pressure = pascal (Pa = N/m²)
- Energy = joule (J = kg·m²/s²)
JEE Tip: Learn dimensional formulas for derived quantities; they help cross-check equations and eliminate options in multiple-choice questions.
Unit Conversions
Students must practice converting between systems — for example, from CGS to SI:
1 dyne = 10⁻⁵ N, 1 erg = 10⁻⁷ J, 1 cm³ = 10⁻⁶ m³.
Mass and Weight
Mass
Mass is a measure of the amount of matter in an object and does not change with location. It reflects the inertia of the object.
- SI Unit: kilogram (kg)
- Measurement Tools: Analytical balance, beam balance, or electronic balance.
Example: A 2 kg sample of sodium remains 2 kg whether it’s on Earth or the Moon.
Weight
Weight is the gravitational force acting on an object:
Weight = Mass × Acceleration due to gravity (g)
- SI Unit: newton (N)
- Example: A 10 kg object on Earth weighs 98 N (using g = 9.8 m/s²).
Conceptual Link: On the Moon (g ≈ 1.63 m/s²), the same object would weigh only 16.3 N, though its mass remains 10 kg.
JEE Application: Weight variation affects buoyancy and pressure, which appear in thermodynamic and physical problems.
Volume
Volume measures the amount of space occupied by matter.
- SI Unit: cubic meter (m³)
- Other Common Units: litre (L), millilitre (mL), and cubic centimetre (cm³).
Conversion Relations:
1 L = 10⁻³ m³ = 1000 mL = 1000 cm³
Formulas:
- Volume of a cube = a³
- Volume of a cylinder = πr²h
- Volume of a sphere = 4/3 πr³
Measurement Instruments: Volumetric flasks, pipettes, and burettes ensure high precision in laboratory setups.
Example Problem: If a gas occupies 2.24 L at STP, find its volume at 300 K and 1 atm. Using the ideal gas law, V₂ = V₁ × (T₂/T₁) = 2.24 × (300/273) = 2.46 L.
Practical Connection: Volume plays a role in determining molarity and concentration, both key concepts in physical and analytical chemistry.
Density
Density quantifies how much mass is contained in a given volume:
Density = Mass / Volume
SI Unit: kg/m³ (also expressed as g/cm³ for solids and liquids)
Example: A cube of aluminium (mass = 270 g, volume = 100 cm³) has a density of 2.7 g/cm³.
Extended Example: If two liquids (A and B) have densities of 1.0 g/cm³ and 0.8 g/cm³ respectively, and they are mixed, the lighter liquid (B) floats. This understanding forms the basis for fluid mechanics and solutions.
Industrial Applications: Determining metal purity, oil-water separation, and designing ships or submarines.
JEE Tip: Combine density formulas with mole concept and ideal gas equations for advanced problem-solving.
Temperature
Temperature measures the average kinetic energy of particles in a substance. It dictates the direction of heat flow and is crucial for thermodynamics.
Temperature Scales
| Scale | Unit | Relation |
| Celsius | °C | – |
| Kelvin | K | K = °C + 273.15 |
| Fahrenheit | °F | °F = (9/5 × °C) + 32 |
Example: Convert 50°C to K: 50 + 273 = 323 K.
Important Note: Kelvin is used in all JEE calculations as it is absolute — 0 K means complete absence of molecular motion.
Devices for Measurement
- Thermometers: Mercury, alcohol, and digital thermometers.
- Thermocouples: Used for high-temperature measurement in labs and industries.
Influence on Reactions
- Reaction rate increases with temperature due to higher molecular kinetic energy.
- Affects gas expansion, diffusion rates, and equilibrium shifts.
JEE Integration: Problems related to gas laws, reaction kinetics, and entropy all use temperature as a core variable.
Insights for JEE
- Dimensional Analysis: Verifies equation consistency. Example: Check if PV = nRT is dimensionally correct.
- Significant Figures: Master rounding-off rules for experimental data.
- Linked Concepts: Thermodynamics, Gaseous State, and Chemical Kinetics.
- JEE Weightage: ~2–3 marks; however, its principles extend across multiple chapters.
Sample Conversion: Convert 3.6 g/cm³ to kg/m³.
3.6 × 10³ = 3600 kg/m³.
Advanced Example: If density = 8.96 g/cm³ and volume = 10 cm³, mass = 89.6 g.
FAQs
Q1. What distinguishes physical from chemical properties?
Physical properties can be measured without altering chemical identity, while chemical properties involve transformation into new substances.
Q2. Why is the SI system universally used?
It ensures uniformity and comparability across experiments and simplifies scientific communication.
Q3. What’s the difference between accuracy and precision?
Accuracy shows closeness to the true value, while precision shows repeatability among observations.
Q4. How does temperature affect measurement accuracy?
Temperature changes can cause expansion or contraction of measuring instruments, influencing readings.
Q5. Why is Kelvin the preferred scale in chemistry?
Kelvin directly relates to particle energy and avoids negative values, making it ideal for theoretical and numerical calculations.
Q6. What are common sources of measurement error?
Instrumental error, parallax error, and environmental factors like humidity or temperature.
Q7. How can you improve experimental accuracy?
Use calibrated equipment, take multiple readings, avoid parallax, and perform experiments under controlled conditions.
Q8. Why is density crucial in practical chemistry?
It helps identify unknown substances, calculate concentrations, and determine material purity.
Conclusion
The properties of matter and their measurement serve as a foundation for nearly every concept in chemistry. For JEE aspirants, this topic develops both analytical and numerical reasoning skills. Understanding how matter behaves, how it can be measured accurately, and how its units interrelate prepares students for complex topics like thermodynamics, stoichiometry, and kinetics. By practicing unit conversions, applying dimensional analysis, and mastering physical and chemical distinctions, students strengthen their ability to tackle high-level chemistry problems confidently and precisely.





Get Social