Isomerism is one of the most fascinating and essential ideas in organic chemistry. It explains why compounds with the same molecular formula can behave entirely differently due to variations in structure or spatial arrangement. NCERT Class 11 highlights Isomerism because it forms the foundation for advanced topics such as stereochemistry, reaction mechanisms, molecular geometry, and pharmaceutical chemistry.
At Deeksha Vedantu, we help students understand isomerism through visual diagrams, structural mapping, molecular modelling, and systematic explanations. This ensures that students recognize the subtle ways in which molecules can differ-and how these differences influence chemical behaviour.
What Is Isomerism?
Isomerism occurs when two or more compounds share the same molecular formula but differ in either:
- The connectivity of atoms (structural isomers), or
- The three-dimensional arrangement of atoms (stereoisomers).
Such compounds are known as isomers, and they often differ in boiling point, melting point, solubility, reactivity, colour, optical activity, and even smell.
For example, C₅H₁₂ has three distinct structural forms with dramatically different properties. Even more striking is how optical isomers of medicines can have opposite biological effects.
Why Is Isomerism Important?
Understanding isomerism helps students:
- Analyse molecular structure precisely
- Predict physical and chemical behaviour
- Understand reaction pathways
- Make connections between structure and function
- Build strong fundamentals for JEE, NEET, and competitive exams
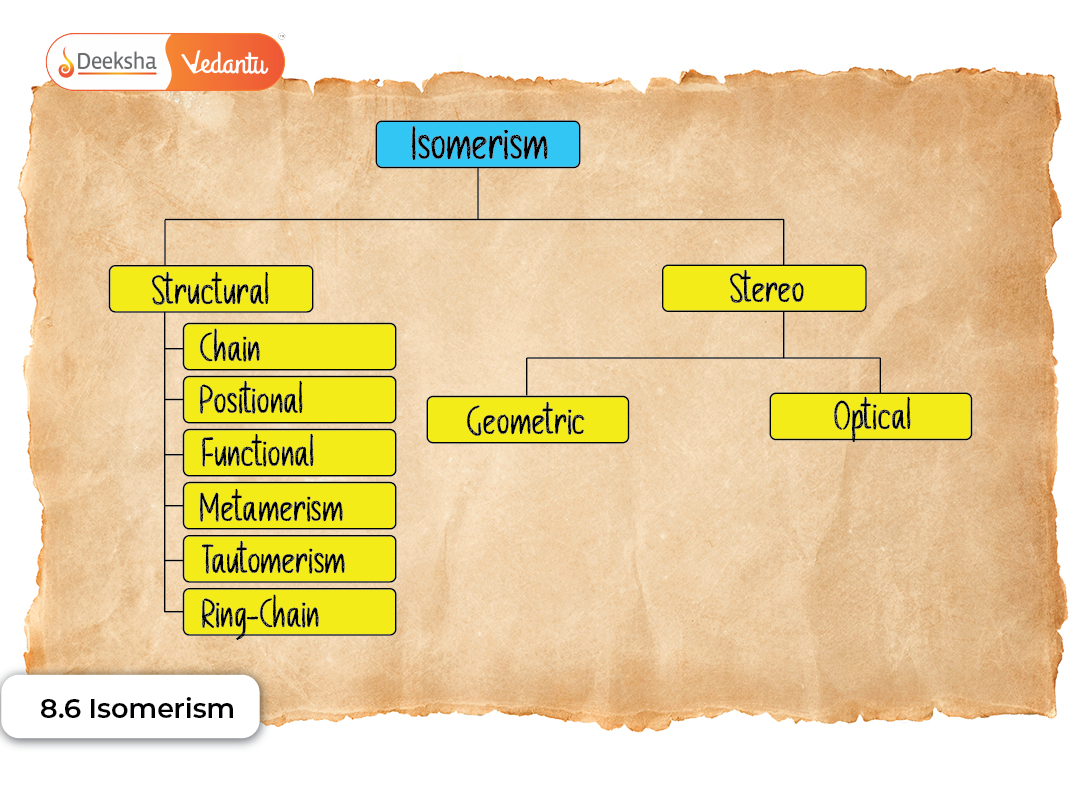
Main Types of Isomerism
Isomerism is divided into two broad categories:
- Structural Isomerism
- Stereoisomerism
Each category contains multiple sub-types that reflect unique ways molecules can differ.
Structural Isomerism
Structural isomerism occurs when molecules share the same molecular formula but differ in the order in which atoms are connected. These differences often create compounds with entirely different physical and chemical properties.
NCERT identifies several types of structural isomerism:
Chain Isomerism
Chain isomers differ in the carbon chain arrangement-straight chain or branched.
Example (C₅H₁₂):
- n-pentane → straight chain
- isopentane → one branch
- neopentane → highly compact, two branches
These variations influence boiling point, density, and combustion characteristics.
Position Isomerism
Position isomers have the same carbon skeleton and functional group but differ in the position of substituents or multiple bonds.
Examples:
- 1-bromopropane vs 2-bromopropane
- But-1-ene vs but-2-ene
Even a shift of one carbon can change reactivity patterns significantly.
Functional Group Isomerism
In this type, compounds have the same molecular formula but different functional groups.
Examples (C₃H₆O):
- Propanal (aldehyde)
- Propanone (ketone)
Because functional groups dictate reactions, these isomers show drastic behavioural differences.
Metamerism
Metamerism arises when compounds contain different alkyl groups on either side of a polyvalent atom such as oxygen (ethers) or nitrogen (amines).
Example:
- Ethoxypropane
- Methoxybutane
Although both share the same molecular formula, their alkyl group distribution changes chemical properties.
Tautomerism
Tautomers are special isomers that exist in a dynamic equilibrium due to internal proton shifts.
Example:
- Keto–enol tautomerism
While NCERT introduces this lightly in Class 11, the phenomenon becomes important in Class 12 organic chemistry and biochemistry.
Stereoisomerism
Stereoisomerism occurs when molecules have the same structural formula but differ in their three-dimensional spatial arrangement.
NCERT divides stereoisomerism into:
- Geometrical Isomerism
- Optical Isomerism
These variations often influence how molecules interact with enzymes, receptors, and light.
Geometrical Isomerism (cis/trans)
Geometrical isomerism usually occurs due to restricted rotation around a double bond (C=C) or within a cyclic structure.
Conditions for Geometrical Isomerism
- A C=C double bond
- Each carbon in the double bond is bonded to two different substituents
Types:
- cis form → similar groups on the same side
- trans form → similar groups on opposite sides
Examples:
- cis-but-2-ene
- trans-but-2-ene
These isomers differ in polarity, boiling point, stability, and even dipole moment.
Importance in Industry:
- cis and trans fats affect health differently
- polymer structures depend on geometrical arrangement
Optical Isomerism
Optical isomerism arises when a molecule contains a chiral carbon atom-a carbon bonded to four different groups.
Key ideas:
- Optical isomers are mirror images called enantiomers.
- They cannot be superimposed on each other.
- They rotate plane-polarised light:
- (+) → dextrorotatory (clockwise)
- (−) → levorotatory (anticlockwise)
Example:
- Lactic acid exists in two chiral forms, each interacting differently with biological systems.
Applications:
- Pharmaceuticals must use the correct enantiomer for safe drug activity.
- Many biomolecules (amino acids, sugars) are chiral.
Structural vs Stereoisomerism
| Feature | Structural Isomerism | Stereoisomerism |
| Basis | Connectivity varies | Spatial orientation varies |
| Types | Chain, position, functional, metamerism, tautomerism | Geometrical, optical |
| Isomer count | Often higher due to multiple bonding patterns | Limited by spatial constraints |
| Example | Butan-1-ol vs 2-methylpropan-1-ol | cis/trans but-2-ene, lactic acid enantiomers |
| Impact | Alters functional behaviour | Alters light rotation & biological response |
Real‑Life Importance of Isomerism
Isomerism plays a crucial role across science, industry, and daily life. Even though isomers share the same molecular formula, their different structures or spatial orientations lead to significantly varied behaviours, making isomerism a key concept in fields ranging from medicine to environmental science.
Medicine
Enantiomers often behave differently inside the human body because biological receptors are themselves chiral. This means the body can distinguish between left‑handed and right‑handed molecules.
- One enantiomer of a drug may be therapeutic, while the other may be inactive or harmful.
- Example: Ibuprofen is sold as a racemic mixture, but only one enantiomer is biologically active.
- In drug design, identifying the correct enantiomer is crucial for safety and effectiveness.
Perfumery & Flavours
Many fragrance and flavour molecules exist as optical isomers, and each isomer can trigger a different sensory experience.
- Example: One isomer of carvone smells like spearmint, while the other smells like caraway.
- Isomerism helps industries craft specific aromas, flavours, and fragrances with precision.
Polymer Chemistry
Isomeric arrangement of monomers affects the structure, strength, and flexibility of polymers.
- Tacticity (arrangement of side groups) determines whether a polymer is flexible, rigid, or crystalline.
- Geometrical isomerism influences polymer stability and melting point.
- Example: cis‑polyisoprene (natural rubber) is elastic, while trans‑polyisoprene (gutta‑percha) is hard.
Food Chemistry
cis and trans geometrical isomers influence nutritional value, digestion, and metabolic impact.
- cis‑fats are typically naturally occurring and healthier.
- trans‑fats (often produced through hydrogenation) are associated with health risks.
- Isomers of sugars, such as glucose and galactose, differ only in orientation but behave very differently in metabolism.
Environmental & Biological Systems
Enzymes, hormones, DNA, and proteins are chiral; hence they interact selectively with specific isomers.
- This selectivity determines how nutrients are absorbed.
- Environmental degradation of chemicals can vary depending on isomeric form.
Petroleum Industry
The degree of branching in hydrocarbons influences the efficiency and cleanliness of fuel combustion.
- Branched‑chain isomers have higher octane numbers than straight‑chain isomers.
- They burn more efficiently, reduce knocking in engines, and improve fuel performance.
Deeksha Vedantu uses 3D models, interactive simulations, and visualisation tools to help students grasp how these subtle structural changes lead to powerful differences in real‑world behaviour.
FAQs
Q1. What causes isomerism in organic molecules?
Isomerism occurs because atoms can rearrange structurally or spatially while keeping the same molecular formula.
Q2. How do chain isomers differ from position isomers?
Chain isomers vary in carbon skeleton branching, while position isomers differ in the location of substituents or functional groups.
Q3. What is the main requirement for geometrical isomerism?
Restricted rotation-usually due to a double bond or ring structure.
Q4. Why do optical isomers rotate light differently?
Because they are chiral and interact differently with plane-polarised light.
Q5. Can two compounds with different functional groups be isomers?
Yes, functional group isomers share a molecular formula but possess different functional groups.
Conclusion
Isomerism brings diversity, complexity, and richness to organic chemistry. Small structural or spatial changes can produce compounds with entirely different properties, reactivities, or biological activities.
At Deeksha Vedantu, we help students decode isomerism using models, examples, comparisons, and structured learning-providing a strong foundation for advanced topics such as stereochemistry, reaction mechanisms, and organic synthesis.






Get Social