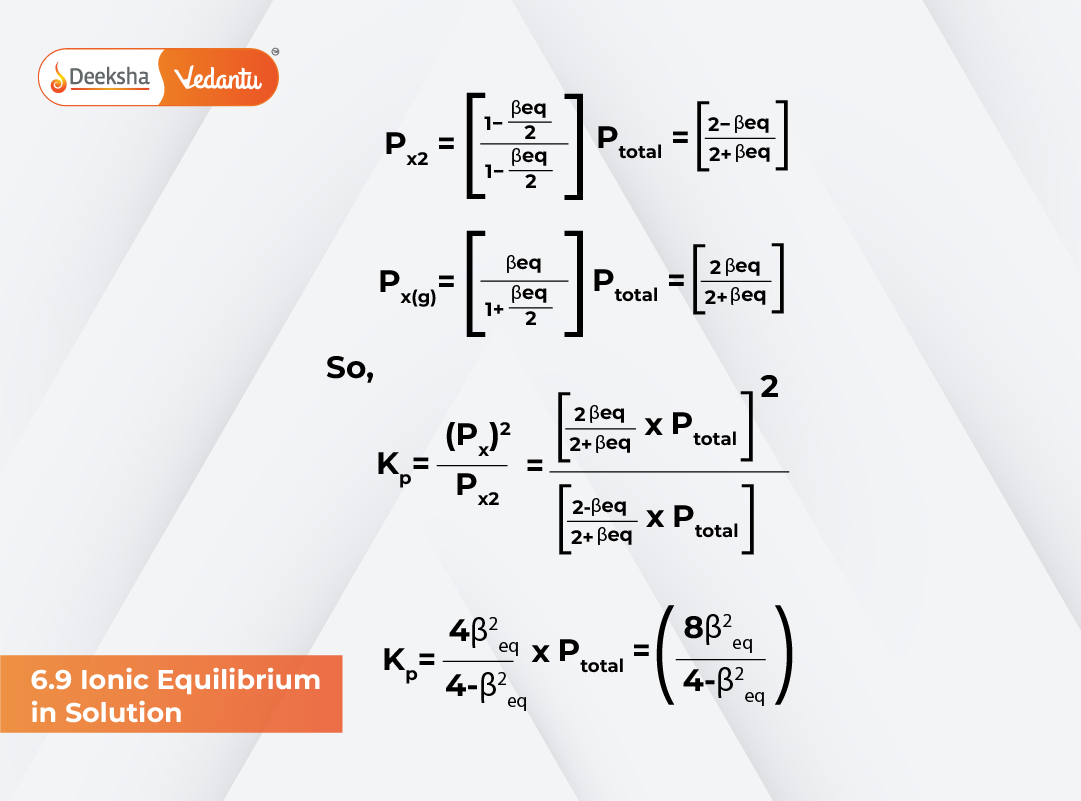
When studying equilibrium, we often deal with reactions involving ions in solution. One common example is:
Fe³⁺(aq) + SCN⁻(aq) ⇌ [Fe(SCN)]²⁺(aq)
This equilibrium demonstrates how ions in solution can form complex species, and how their concentrations are governed by the principles of chemical equilibrium. To fully understand ionic equilibrium, it’s essential to examine how ions behave in aqueous systems and how this differs from molecular systems.
Understanding Ionic Equilibrium
Many equilibria involve ions, especially in aqueous solutions. It is well known that pure water or sugar solution does not conduct electricity, while a solution of salt in water does. This difference arises because salts dissociate into ions in solution, allowing electric current to pass through. The more ions present, the greater the conductivity.
The study of ionic equilibrium deals with such processes-how ions form, interact, and reach equilibrium in a solution.
Faraday’s Classification of Substances
Michael Faraday classified substances into two broad categories based on their ability to conduct electricity:
- Electrolytes: Substances that conduct electricity in their aqueous or molten state. They dissociate into ions, which carry electric current.
- Non-electrolytes: Substances that do not conduct electricity because they remain as neutral molecules in solution.
Faraday further divided electrolytes into two types:
- Strong Electrolytes: Completely ionize in solution, producing a high concentration of ions. Examples include sodium chloride (NaCl) and hydrochloric acid (HCl).
- Weak Electrolytes: Only partially ionize in solution, resulting in an equilibrium between ions and unionized molecules. Examples include acetic acid (CH₃COOH) and ammonium hydroxide (NH₄OH).
Examples of Strong and Weak Electrolytes
Strong Electrolyte Example
When sodium chloride dissolves in water:
NaCl(s) → Na⁺(aq) + Cl⁻(aq)
The dissociation is nearly 100%, meaning almost all sodium chloride molecules are converted into ions. Hence, NaCl is a strong electrolyte.
Weak Electrolyte Example
When acetic acid dissolves in water:
CH₃COOH(aq) ⇌ CH₃COO⁻(aq) + H⁺(aq)
Only about 5% of the molecules ionize; the remaining stay undissociated. Therefore, acetic acid is a weak electrolyte.
Establishment of Ionic Equilibrium
In weak electrolytes, there exists an equilibrium between ions and the unionized molecules. This equilibrium is dynamic in nature-ions continually combine to form undissociated molecules while undissociated molecules ionize to produce ions.
The equilibrium can be represented as:
HA ⇌ H⁺ + A⁻
This type of equilibrium is called Ionic Equilibrium.
The position of ionic equilibrium depends on the degree of ionization and the concentration of ions in solution. Factors such as dilution, temperature, and the presence of a common ion also affect this equilibrium.
Conductivity and Ionization
The ability of a solution to conduct electricity directly depends on the number of ions present. Thus:
- Strong electrolytes show high electrical conductivity because they produce a large number of ions.
- Weak electrolytes show low conductivity since only a small fraction of their molecules ionize.
This concept is crucial for understanding acid-base chemistry, salt hydrolysis, and buffer systems, all of which depend on ionic equilibria.
Key Differences Between Strong and Weak Electrolytes
| Feature | Strong Electrolytes | Weak Electrolytes |
| Degree of Ionization | Nearly 100% | Less than 5% |
| Nature of Dissociation | Complete | Partial |
| Examples | NaCl, HCl, KOH | CH₃COOH, NH₄OH |
| Conductivity | Very high | Low |
| Dependence on Dilution | Little effect | Ionization increases with dilution |
Importance of Ionic Equilibrium
Understanding ionic equilibrium is essential because it:
- Explains the conductivity of solutions.
- Helps determine the strength of acids and bases.
- Lays the foundation for acid-base equilibria, salt hydrolysis, and buffer solutions.
- It is vital in analytical chemistry and biochemical systems, where ionic concentrations determine reaction outcomes.
Conclusion
Ionic equilibrium represents the balance between ionized and unionized forms of a substance in solution. This equilibrium governs the behavior of acids, bases, and salts in aqueous media. Substances that ionize completely are strong electrolytes, while those that ionize partially are weak electrolytes. Understanding ionic equilibrium is key to mastering topics such as acid-base balance, pH calculation, titration curves, and buffer action – all of which are crucial for NEET and JEE chemistry preparation.






Get Social