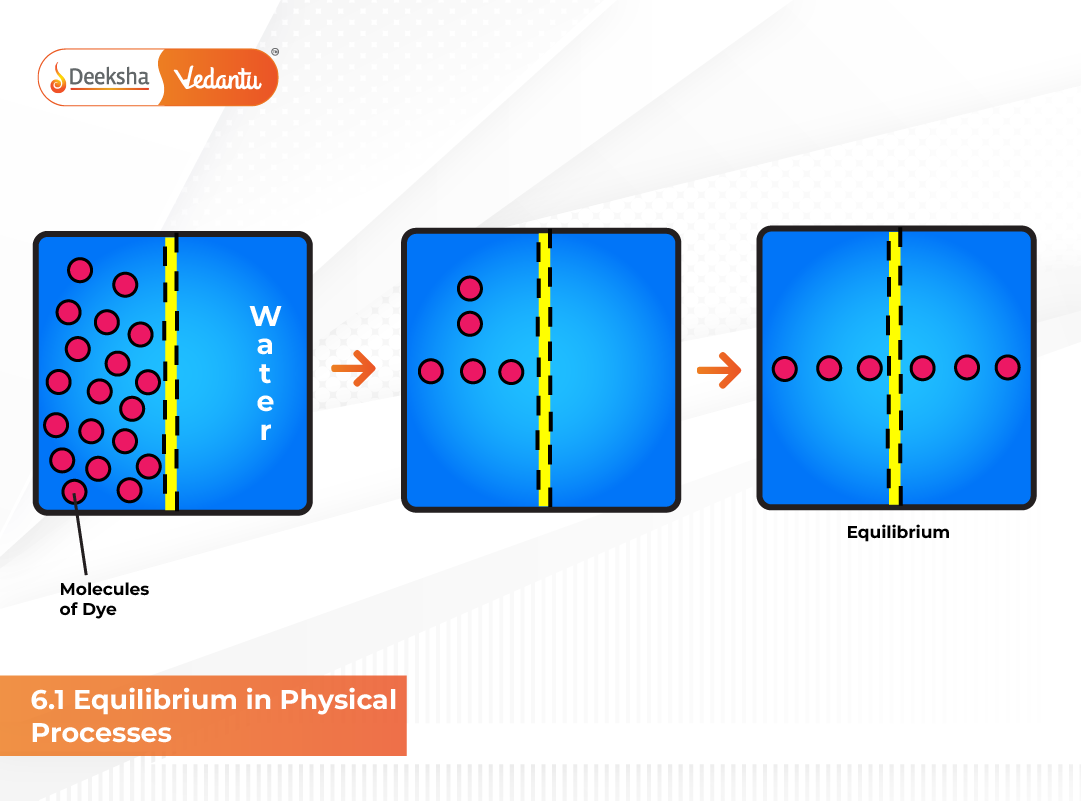
Physical equilibrium refers to the state of balance that occurs during reversible physical changes, where two opposing processes happen simultaneously at equal rates. Such equilibria involve transformations like melting, boiling, or dissolving, where the overall observable properties remain constant. These phenomena highlight that equilibrium can exist even in the absence of chemical reactions. Common examples include the phase changes among solid, liquid, and gaseous states:
Solid ⇌ Liquid ⇌ Gas
At equilibrium, both forward and reverse changes occur at identical rates, leading to a stable and measurable condition. These physical equilibria play a crucial role in understanding thermodynamic principles, vapor pressure relationships, and solubility behavior — all foundational concepts for NEET and JEE aspirants.
Solid–Liquid Equilibrium
A classic example of solid–liquid equilibrium is the system of ice and water at 0°C under 1 atm pressure. In a perfectly insulated environment, when ice and water coexist, the rate at which ice melts is exactly balanced by the rate at which water freezes. Hence, the system achieves equilibrium.
H₂O(s) ⇌ H₂O(l)
This equilibrium is temperature- and pressure-dependent. An increase in pressure or decrease in temperature favors the solid phase, while the opposite conditions favor the liquid phase. This explains why ice melts faster under pressure — a principle that helps skaters glide smoothly on ice, as the pressure exerted by the blade lowers the melting point of ice, forming a thin layer of water that acts as a lubricant.
At equilibrium, there is no net change in the amount of ice or water, but both melting and freezing processes continue at equal rates. This dynamic balance is a hallmark of all equilibrium states.
Important for NEET/JEE: Solid–liquid equilibrium emphasizes how physical states depend on temperature and pressure variations and demonstrates the dynamic nature of equilibrium — a key topic in thermodynamics.
Liquid–Vapour Equilibrium
Consider water in a closed container at a fixed temperature. Initially, water molecules evaporate into the space above the liquid, increasing the vapor pressure. As the vapor concentration grows, some molecules begin to condense back into liquid. When the rates of evaporation and condensation become equal, the system reaches liquid–vapour equilibrium.
H₂O(l) ⇌ H₂O(g)
At equilibrium, the vapor pressure remains constant at a given temperature. This steady value is known as equilibrium vapor pressure or saturated vapor pressure. The concept of vapor pressure is crucial in explaining phenomena like boiling, humidity, and cloud formation.
Factors Affecting Liquid–Vapour Equilibrium
- Temperature: Increasing temperature raises the kinetic energy of molecules, enhancing evaporation and thus increasing vapor pressure.
- Nature of Liquid: Liquids with weaker intermolecular forces (like ether or alcohol) have higher vapor pressures than those with stronger forces (like water).
- External Pressure: When vapor pressure equals the external atmospheric pressure, the liquid boils. This temperature is called the boiling point. At higher altitudes, atmospheric pressure is lower, so liquids boil at lower temperatures.
Equation:
H₂O(l) ⇌ H₂O(g), ΔH > 0 (endothermic process)
At 100°C and 1.013 bar pressure, water boils in an open container. In a closed system, equilibrium is reached before the boiling point, where the vapor pressure stabilizes at a constant value.
Practical Application: The study of liquid–vapour equilibrium helps in designing distillation columns, understanding cloud condensation, and calculating enthalpy of vaporization.
Solid–Vapour Equilibrium
Solid–vapour equilibrium occurs in substances that undergo sublimation, meaning they transition directly from solid to vapor without passing through a liquid phase. Equilibrium is attained when the rate of sublimation equals the rate of deposition (condensation of vapor back into solid form).
Examples:
- Camphor(s) ⇌ Camphor(g)
- NH₄Cl(s) ⇌ NH₃(g) + HCl(g)
In a sealed container, the vapor pressure above the solid increases as sublimation proceeds. Eventually, a steady state is reached where the number of molecules returning to the solid phase equals those escaping from it. The vapor pressure at this point remains constant for a given temperature but rises when the temperature increases.
Significance: Such equilibria illustrate how solids like camphor or iodine slowly disappear when exposed to air due to sublimation, a concept tested in both theoretical and experimental questions.
Equilibrium Involving Dissolution of Solids or Gases in Liquids
Equilibria also exist when solids or gases dissolve in liquids. These systems highlight how solubility and external conditions influence equilibrium states.
(a) Solids in Liquids
When a solid like sugar or salt dissolves in water, a dynamic equilibrium is established between the undissolved solid and its dissolved ions or molecules.
NaCl(s) ⇌ Na⁺(aq) + Cl⁻(aq)
The rate of dissolution equals the rate of crystallization, maintaining a constant concentration of solute in a saturated solution. Temperature plays a crucial role — generally, solubility of solids increases with temperature because higher kinetic energy allows more solute particles to overcome intermolecular forces.
In NEET and JEE contexts, this principle is often tested through solubility products (Ksp) and crystallization-based questions.
(b) Gases in Liquids
When a gas dissolves in a liquid, equilibrium is achieved between the gas molecules in the liquid phase and those in the gaseous phase above it.
CO₂(g) ⇌ CO₂(aq)
This equilibrium is quantitatively described by Henry’s Law, which states that:
p = kₕx
where:
- p = partial pressure of the gas,
- x = mole fraction of the gas in the solution,
- kₕ = Henry’s Law constant (unique for each gas–solvent pair).
Applications:
- Carbonated beverages are bottled under high pressure to maintain CO₂ solubility. Opening the bottle decreases pressure, causing gas bubbles to escape.
- Aquatic life depends on the solubility of oxygen in water, which decreases with rising temperature.
NEET/JEE Tip: Henry’s Law helps in solving numerical problems on gas solubility, diving physiology, and pressure dependence in solution equilibria.
General Characteristics of Equilibria Involving Physical Processes
The following properties are common to all physical equilibria:
- Equilibrium is attained only in a closed system at a specific temperature.
- Opposing processes occur simultaneously and continuously, maintaining a dynamic balance.
- Measurable quantities such as pressure, temperature, and concentration remain constant once equilibrium is established.
- The equilibrium state is identified by fixed values of measurable parameters like vapor pressure or solute concentration.
- The magnitude of equilibrium parameters reflects the extent to which the process has advanced before reaching balance.
- Physical equilibria are reversible, meaning that any disturbance (change in temperature or pressure) can shift the equilibrium position according to Le Chatelier’s Principle.
Key Features of Physical Equilibria
| Process | Expression at Equilibrium | Constant Property | Explanation |
| Liquid ⇌ Vapour | H₂O(l) ⇌ H₂O(g) | p₍vapour₎ constant at given T | Represents evaporation–condensation balance |
| Solid ⇌ Liquid | Ice ⇌ Water | Melting point fixed at 273 K (1 atm) | Energy absorbed = energy released |
| Solute(s) ⇌ Solution | NaCl(s) ⇌ Na⁺ + Cl⁻ | Concentration constant at saturation | Dissolution and crystallization rates equal |
| Gas ⇌ Gas (in solution) | CO₂(g) ⇌ CO₂(aq) | p₍gas₎/x constant (Henry’s Law) | Solubility directly proportional to pressure |
NEET & JEE Insights
- Solid–liquid equilibrium highlights the correlation between phase transition and energy conservation.
- Vapor pressure relationships help solve advanced numerical questions on boiling points and phase diagrams.
- Henry’s Law and solubility equilibria are frequent topics in physical chemistry sections of NEET and JEE.
- Understanding how external factors affect equilibrium is key to predicting phase behavior under various thermodynamic conditions.
FAQs
Q1. What is equilibrium in physical processes?
It is the state in which two opposing physical changes, such as melting and freezing or evaporation and condensation, occur simultaneously at equal rates.
Q2. What is equilibrium vapor pressure?
It is the pressure exerted by a vapor in equilibrium with its liquid at a specific temperature, indicating the liquid’s volatility.
Q3. How does temperature affect solubility?
For most solids, solubility increases with temperature; however, for gases, solubility decreases because higher temperatures reduce gas retention in the solvent.
Q4. Why are carbonated beverages bottled under pressure?
Because increased pressure enhances the solubility of CO₂ in the liquid, preventing gas escape until the bottle is opened.
Q5. What is dynamic equilibrium?
Dynamic equilibrium is a condition where forward and reverse processes continue at the same rate, keeping macroscopic properties constant.
Q6. What is Henry’s Law and its application?
Henry’s Law states that the mass of gas dissolved in a liquid at a constant temperature is proportional to its partial pressure. It is applied in carbonation, underwater breathing systems, and environmental chemistry.
Conclusion
Physical equilibria provide a framework to understand how natural systems maintain balance between different states of matter. From melting ice to vapor formation, these processes illustrate the harmony between opposing molecular motions. A strong grasp of these concepts enables students to analyze real-world situations such as humidity control, sublimation of substances, and the behavior of gases under varying pressures and temperatures. Such understanding not only strengthens the theoretical foundation but also enhances performance in NEET, JEE, and other competitive examinations.






Get Social