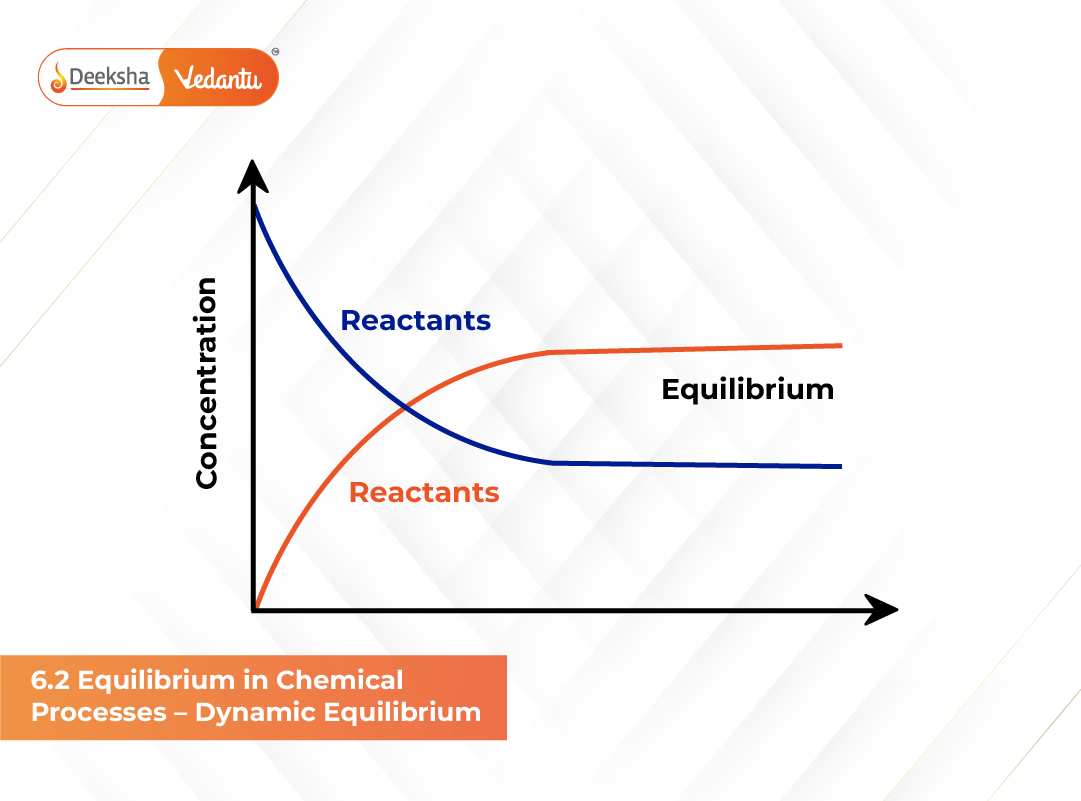
Chemical reactions, like physical systems, can reach a state of balance known as chemical equilibrium, where two opposing reactions – forward and reverse – occur simultaneously. This balance doesn’t imply that reactions stop; instead, they continue at equal rates, leading to a stable concentration of reactants and products. This type of balance is called dynamic equilibrium.
Dynamic equilibrium plays a fundamental role in understanding how chemical systems behave under different conditions. It explains why reactions that seem “complete” still involve continuous molecular motion and why certain reversible reactions are key to industrial and biological processes.
Understanding Chemical Equilibrium in Detail
Consider a general reversible reaction:
A + B ⇌ C + D
At the start, the forward reaction dominates because the concentration of reactants (A and B) is high. As products (C and D) form, their concentrations increase while A and B gradually decrease. This change slows down the forward reaction and simultaneously accelerates the reverse reaction, in which products react to regenerate reactants.
Eventually, the rate of the forward reaction becomes equal to the rate of the reverse reaction. This moment marks the state of equilibrium. At this point, there is no observable change in concentrations, though both reactions continue endlessly at the microscopic level.
A fascinating feature of chemical equilibrium is that it can be reached from either side of the reaction. If a system starts with only reactants, products will form until equilibrium is reached. Conversely, if the process starts with only products, they will decompose to form reactants until the same equilibrium composition is achieved. This demonstrates the reversible nature of chemical changes.
Key Insight: Chemical equilibrium is dynamic – the forward and reverse reactions are active, and molecular transformations persist even when concentrations appear constant.
The Dynamic Nature of Chemical Equilibrium
To understand the true nature of equilibrium, we can analyze the Haber process, a well-known industrial method for producing ammonia.
Reaction:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
During this reaction, nitrogen and hydrogen gases combine under high temperature and pressure in the presence of a catalyst. As the reaction proceeds, ammonia forms. If one monitors the system over time, the concentrations of N₂, H₂, and NH₃ eventually become constant – indicating that the system has reached equilibrium.
At equilibrium, molecules of ammonia are simultaneously forming and decomposing, maintaining a constant ratio of products to reactants. Thus, even though the macroscopic appearance of the system doesn’t change, molecular activity continues without interruption.
Demonstrating Dynamic Equilibrium with Isotopes
An elegant proof of equilibrium’s dynamic nature comes from experiments involving isotopes. When a mixture of nitrogen and hydrogen (N₂, H₂) and their isotopic variants (ND₃, D₂) is allowed to reach equilibrium, the resulting ammonia contains mixed isotopic forms such as NH₃, ND₃, NHD₂, and NH₂D.
This observation proves that even after equilibrium has been achieved, exchange between isotopic atoms continues. If equilibrium were static, no mixing of isotopes would occur. Instead, continuous reaction ensures redistribution of hydrogen and deuterium atoms across different ammonia molecules.
Demonstrating Dynamic Equilibrium – A Simple Classroom Activity
Dynamic equilibrium can be demonstrated visually using a simple experimental setup involving diffusion between liquids.
Materials Needed:
- Two measuring cylinders (marked 1 and 2, each 100 mL)
- Two glass tubes (30 cm each)
- Coloured water (using KMnO₄ or ink)
- Connecting tubes for transfer
Procedure:
- Fill one cylinder (Cylinder 1) with coloured water and the other (Cylinder 2) with clear water.
- Connect both using glass tubes, ensuring each tube end is submerged.
- Tilt the setup slightly to allow water to flow from Cylinder 1 to Cylinder 2.
- Allow the system to stand undisturbed.
Initially, there is a net flow of coloured water from Cylinder 1 to Cylinder 2. After some time, the transfer slows down as coloured water starts diffusing back. Eventually, both cylinders have water of the same shade – the dynamic equilibrium point. Molecules still move between the two containers, but their rates are equal, keeping the appearance constant.
Observation: This demonstration shows how equilibrium doesn’t mean stoppage of motion but balance between two opposing processes.
Real-World Analogy: Imagine a crowded hall where people continuously move between two sides, yet the total number of people on each side remains constant. That is dynamic equilibrium in action.
Chemical Equilibrium from Both Directions
Let’s examine another example: the formation of hydrogen iodide (HI).
H₂(g) + I₂(g) ⇌ 2HI(g)
When the reaction begins with equal concentrations of H₂ and I₂, the forward reaction dominates, and HI concentration increases steadily. Over time, as HI builds up, the reverse reaction – decomposition of HI into H₂ and I₂ – intensifies. Eventually, both reactions proceed at the same rate, resulting in equilibrium.
If the process starts with HI instead of H₂ and I₂, decomposition continues until the same equilibrium composition is established. Therefore, regardless of the starting condition, the final equilibrium mixture remains identical.
Important Characteristics of Dynamic Chemical Equilibrium
- Reversibility: Reactions can proceed in both directions under identical conditions.
- Continuity: The forward and reverse reactions continue indefinitely at equal rates.
- Dependence on Conditions: Temperature, pressure, and concentration significantly affect the position of equilibrium.
- Constant Concentrations: Reactant and product concentrations remain steady at equilibrium.
- Equal Reaction Rates: Forward and backward reactions occur simultaneously with identical speeds.
- Dynamic Molecular Activity: Despite no observable changes, molecular exchanges persist.
- Macroscopic Stability: Observable properties like pressure, colour, and volume remain unchanged once equilibrium is reached.
NEET & JEE-Oriented Insights
- The Haber process serves as a central example in equilibrium-related questions, particularly those involving the relationship between reaction rate, pressure, and temperature.
- Graph interpretation: Many JEE and NEET problems feature concentration–time graphs to assess understanding of rate equality.
- Conceptual clarity: Understanding isotopic substitution provides deeper insight into the molecular nature of equilibrium.
- Application relevance: Concepts from this chapter extend to acid-base equilibria, solubility product, and Le Chatelier’s principle – topics heavily tested in exams.
Frequently Asked Questions
Q1. What is dynamic equilibrium?
Dynamic equilibrium is a state in which the forward and reverse reactions of a process occur simultaneously and at equal rates, maintaining constant concentrations of reactants and products.
Q2. Can equilibrium be reached from both reactants and products?
Yes. Regardless of whether the reaction starts with reactants or products, equilibrium composition remains identical, reflecting reversibility.
Q3. What experiment demonstrates the dynamic nature of equilibrium?
The Haber process and isotope exchange experiments (using deuterium) confirm that reactions continue at equilibrium.
Q4. Why is equilibrium described as dynamic?
Because molecular-level exchanges continue even after measurable changes cease.
Q5. How do temperature and pressure affect equilibrium?
Increasing temperature generally favours endothermic reactions, while pressure variations influence reactions involving gaseous molecules.
Q6. What is meant by equal rates in equilibrium?
It means the number of product molecules converting back to reactants per unit time equals the number of reactants forming products in the same period.
Conclusion
The study of dynamic chemical equilibrium forms a bridge between reaction kinetics and thermodynamics. It reveals how chemical systems maintain balance under continuously opposing forces. By understanding this concept, students can better grasp advanced equilibrium principles, calculate equilibrium constants, and predict how systems respond to changes. A clear understanding of equilibrium dynamics empowers learners to approach complex NEET and JEE problems with logic and precision.





Get Social