Organic chemistry includes an enormous range of carbon-containing compounds-so vast that it becomes impossible to study them individually without a strong organisational framework. Classification helps simplify this complexity by grouping compounds with similar structures or behaviours. NCERT Class 11 Chapter 8.4 introduces these systematic ways of classification, enabling students to identify patterns, understand chemical reactivity, and build a strong foundation for more advanced organic chemistry topics.
At Deeksha Vedantu, we focus on helping students understand not just the “what” but also the “why” of classification. When students learn the logic behind grouping compounds, they can easily predict behaviour, solve reaction-based questions, and approach organic chemistry with clarity and confidence.
Why Classification Is Important
With millions of organic compounds, classification becomes an essential learning tool. It allows students to:
- Recognise structural and functional similarities between molecules
- Predict physical properties like boiling point, melting point, and solubility
- Anticipate chemical reactions based on functional groups
- Understand the progression of properties within homologous series
- Organise large amounts of information logically for long-term retention
In organic chemistry, classification acts like a roadmap-helping students navigate from simple to complex ideas with ease.
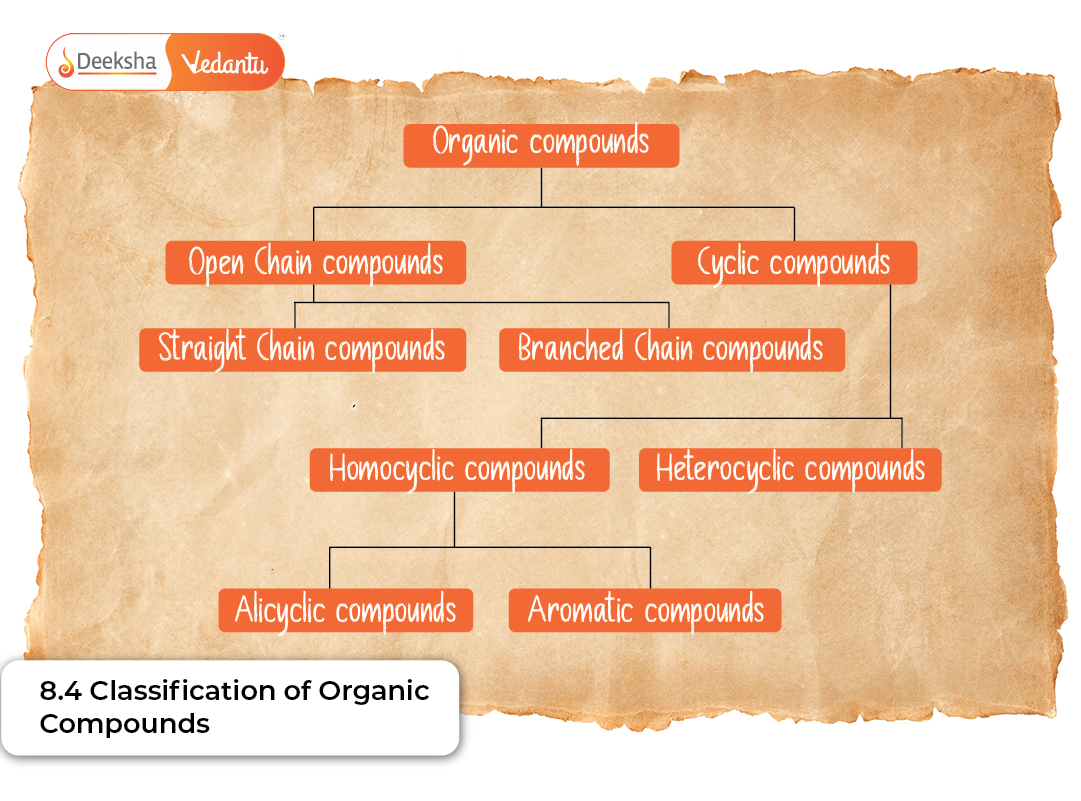
Classification Based on Carbon Chain Structure
The carbon chain acts as the backbone of every organic compound. Depending on how carbons are connected, we can classify compounds into two major types: acyclic and cyclic.
Acyclic (Open-Chain) Compounds
These compounds consist of carbon atoms arranged in straight or branched chains. They are also known as aliphatic compounds.
Examples:
- Hexane – a straight-chain hydrocarbon
- 2-Methylpropane – a branched-chain compound
Acyclic compounds can be:
- Saturated (containing only single bonds) like alkanes
- Unsaturated (containing double or triple bonds) like alkenes and alkynes
Straight and branched chains influence properties such as boiling point and stability. For instance, branching generally lowers boiling point due to reduced surface area.
Cyclic (Closed-Chain) Compounds
Cyclic compounds contain carbon atoms arranged in a ring structure. Depending on the nature of the ring and the atoms present, cyclic compounds can be further classified.
Types of cyclic compounds:
- Alicyclic compounds such as cyclohexane, which resemble aliphatic compounds but have ring structures
- Aromatic compounds like benzene, which contain special delocalised π-electron systems
- Heterocyclic compounds where rings include atoms such as nitrogen, oxygen, or sulfur (e.g., pyridine, furan, thiophene)
Cyclic structures often display unique reactivity due to ring strain, aromatic stability, or heteroatom behaviour.
Classification Based on Functional Groups
Functional groups determine the characteristic reactions of organic compounds. A functional group is a specific atom or group of atoms whose presence defines the chemical properties of that molecule.
Common functional groups include:
- Alcohols (–OH)
- Aldehydes (–CHO)
- Ketones (>C=O)
- Carboxylic acids (–COOH)
- Esters (–COOR)
- Amines (–NH₂)
- Halides (–X, where X = Cl, Br, I)
This classification is extremely valuable because compounds with the same functional group show predictable patterns of behaviour. For example, all alcohols undergo oxidation reactions, and all carboxylic acids show acidic behaviour.
Homologous Series
A homologous series is a family of organic compounds with:
- The same functional group
- A constant difference of –CH₂– between successive members
- Similar chemical behaviour
- Gradual variation in physical properties
Examples:
- Alkanes: CH₄, C₂H₆, C₃H₈, C₄H₁₀
- Alcohols: CH₃OH, C₂H₅OH, C₃H₇OH
Homologous series follow general formulas like:
- Alkanes: CₙH₂ₙ₊₂
- Alkenes: CₙH₂ₙ
- Alkynes: CₙH₂ₙ₋₂
Physical properties such as boiling point increase steadily with chain length due to stronger van der Waals forces.
Classification Based on Degree of Saturation
The presence or absence of multiple bonds in the carbon chain helps classify compounds as saturated or unsaturated.
Saturated Compounds
Contain only single (sigma) bonds. Examples:
- Methane (CH₄)
- Ethane (C₂H₆)
Saturated compounds tend to be less reactive because sigma bonds are strong and stable.
Unsaturated Compounds
Contain one or more double or triple bonds. Examples:
- Ethene (C₂H₄) – a double bond
- Ethyne (C₂H₂) – a triple bond
Unsaturated compounds show higher reactivity due to the presence of π bonds, which are weaker and more reactive.
Classification Based on Aromaticity
Aromatic compounds contain cyclic, planar structures with delocalised π electrons. They follow Huckel’s rule, which states that aromatic rings have 4n + 2 π electrons.
Examples:
- Benzene (C₆H₆)
- Toluene (C₇H₈)
These compounds exhibit special stability known as aromatic stabilisation and typically undergo electrophilic substitution reactions rather than addition reactions.
Classification Based on the Presence of Heteroatoms
Compounds that contain atoms other than carbon and hydrogen in their structure are called heteroatomic compounds.
Examples:
- Alcohols (O)
- Amines (N)
- Organosulfur compounds (S)
- Heterocyclic rings such as furan (O) and pyridine (N)
Heteroatoms affect:
- Polarity
- Hydrogen bonding
- Solubility
- Reactivity patterns
They also introduce new functional groups and reaction pathways.
Examples of Each Classification Category
Example 1: Acyclic Compound
Butane (C₄H₁₀) – a straight-chain hydrocarbon used as fuel. As a member of the alkane family, it burns cleanly and releases energy, making it useful in LPG cylinders. Its open-chain structure influences its boiling point and physical behaviour, and comparing it with isobutane helps students understand the impact of branching.
Example 2: Cyclic Compound
Cyclopentane – a five-membered ring used in industrial solvents. Its ring structure reduces flexibility compared to open-chain counterparts, affecting its reactivity and physical properties. Its structure also helps introduce the concept of ring strain and conformational stability.
Example 3: Aromatic Compound
Benzene (C₆H₆) – the basis for many dyes, pharmaceuticals, and polymers. Its delocalised π-electron system provides exceptional stability, explaining why benzene undergoes substitution reactions instead of addition reactions. Its unique properties form the basis of aromatic chemistry.
Example 4: Functional Group-Based Compound
Ethanol (C₂H₅OH) – an alcohol commonly used in beverages, disinfectants, and fuels. The –OH functional group imparts polarity, allowing hydrogen bonding and influencing solubility. These properties explain its widespread use as a solvent and antiseptic.
Example 5: Homologous Series
Propane → Butane → Pentane – each larger by a –CH₂– group and showing predictable trends. As chain length increases, boiling points rise due to stronger intermolecular forces. The gradual, systematic change in properties helps students understand patterns across a family of compounds.
Real-Life Importance of Classification
Classification helps students understand the behaviour of compounds in real-world applications:
- Hydrocarbons used as fuels differ in combustion behaviour based on saturation.
- Aromatic compounds form the basis of dyes, explosives, and pharmaceuticals.
- Alcohols dissolve in water due to hydrogen bonding, unlike hydrocarbons.
- Drug molecules are grouped by functional groups that influence biological activity.
With classification, students can quickly determine how a compound will behave in experiments, industrial processes, or biological systems.
FAQs
Q1. What is the simplest method for classifying organic compounds?
The simplest method is based on carbon chain structure-acyclic vs. cyclic.
Q2. What is a functional group and why is it important?
A functional group determines the chemical reactivity and characteristic reactions of an organic molecule.
Q3. How does the homologous series help in studying organic chemistry?
It reveals trends in physical properties and allows prediction of behaviour across related compounds.
Q4. What is the difference between saturated and unsaturated compounds?
Saturated compounds contain only single bonds, while unsaturated compounds contain double or triple bonds.
Q5. Why are aromatic compounds considered special?
Aromatic compounds have delocalised π electrons that provide exceptional stability and unique reaction patterns.
Conclusion
Classification of organic compounds creates the essential framework upon which all organic chemistry is built. By grouping compounds according to chain structure, functional groups, saturation, aromaticity, and heteroatom presence, students gain clarity, make accurate predictions, and develop strong analytical skills.
At Deeksha Vedantu, we help learners grasp these patterns through guided learning, visual mapping, and continuous practice-ensuring they confidently progress to advanced topics like isomerism, reaction mechanisms, and stereochemistry.





Get Social