The periodic table is more than a static chart of elements – it’s a dynamic map of chemical behavior. It organizes all known elements in a meaningful way, enabling scientists, educators, and students to recognize patterns and predict properties. These recurring patterns, known as periodic trends or periodicity, reveal how elements behave and interact based on their atomic structure. Understanding these patterns is fundamental to mastering modern chemistry.
In this comprehensive blog, we’ll explore the core aspects of periodicity in the periodic table. You’ll gain insights into major periodic trends such as the atomic radius trend, electronegativity trend, ionization energy trend, and valency patterns. Along the way, we’ll highlight key explanations for why these trends occur, supported by relevant examples and visual aids to help cement your understanding. This guide is particularly useful for Class 10 and 11 students preparing for board exams and competitive entrances.
What Is Periodicity?
Periodicity refers to the systematic and predictable changes in element properties as you move across periods (horizontal rows) or down groups (vertical columns) of the periodic table. These trends are largely a result of the electron configurations of the elements, particularly the arrangement of electrons in their outermost (valence) shells.
The modern periodic table is arranged by increasing atomic number, which defines the number of protons in an atom. This reordering from Mendeleev’s earlier design (which used atomic mass) brought clarity to many inconsistencies and made periodic trends more accurate.
Explore: Modern Periodic Table
An essential factor that contributes to periodic trends is the distribution of electrons in various orbitals. The electronic configuration of the first 30 elements provides a great starting point to see how elements begin filling their shells and how similar configurations lead to repeating patterns.
Understand: Electronic Configuration of First 30 Elements
Key Periodic Trends and Their Explanation
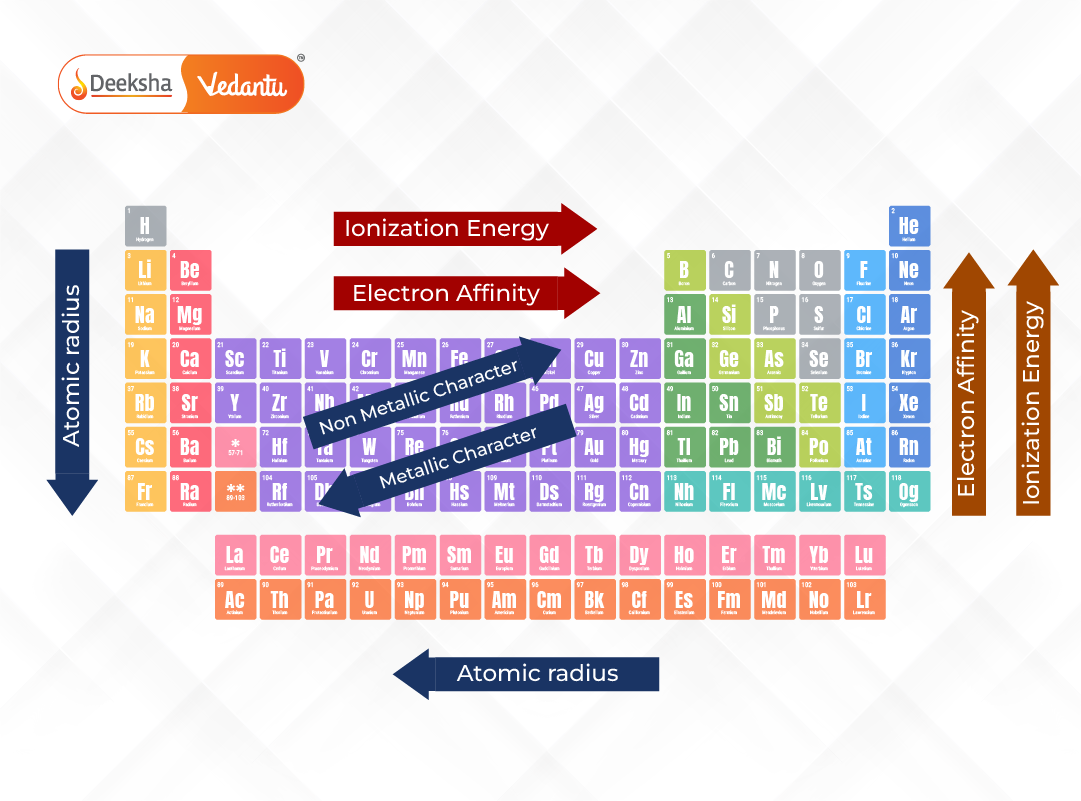
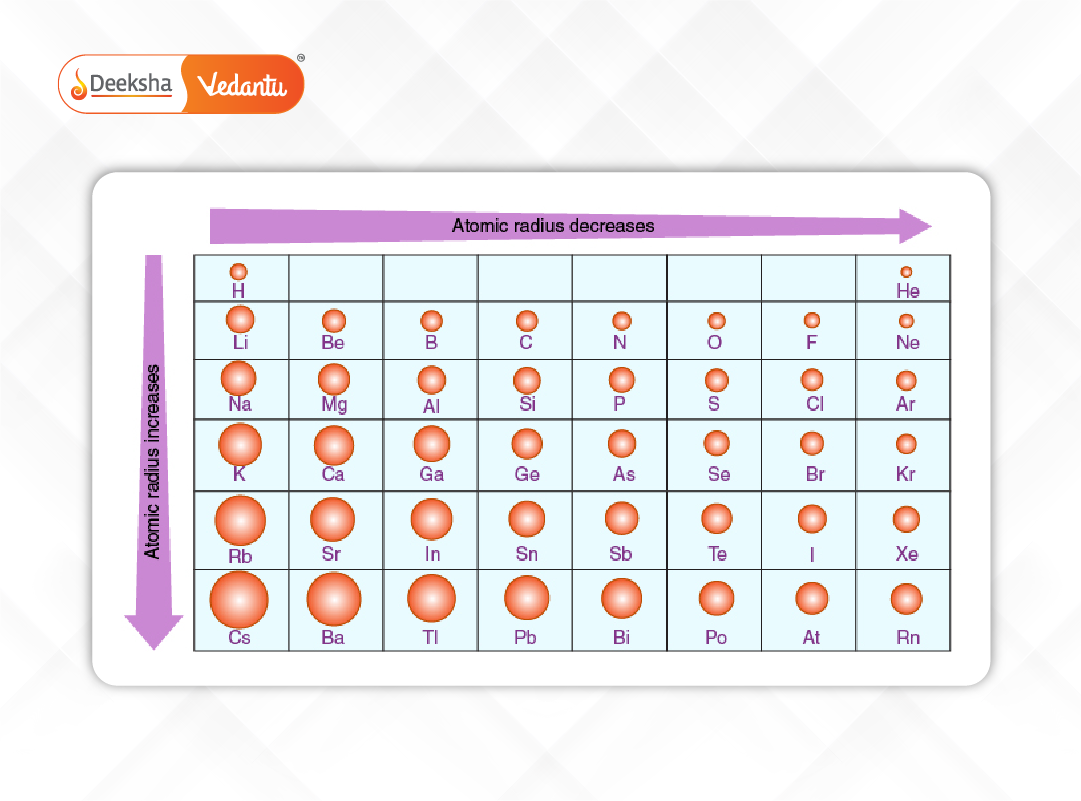
1. Atomic Radius Trend
Atomic radius is defined as the distance from the nucleus to the boundary of the surrounding cloud of electrons. It reflects the size of an atom and helps in predicting how atoms will bond.
- Across a Period: Atomic radius generally decreases from left to right. This is because additional protons increase the nuclear charge, pulling electrons closer and compacting the atom.
- Down a Group: Atomic radius increases. Each successive element adds a new energy level (shell), making the atom larger despite the increasing nuclear charge.
This trend is critical in understanding how atoms interact, form bonds, and influence molecular structures.
2. Ionization Energy Trend
Ionization energy is the minimum amount of energy required to remove the most loosely held electron from an isolated gaseous atom. It indicates how strongly an atom holds onto its electrons.
- Across a Period: Ionization energy increases. Stronger nuclear attraction holds electrons more tightly, making them harder to remove.
- Down a Group: Ionization energy decreases. Outer electrons are farther from the nucleus and are shielded by inner shells, making them easier to remove.
Learn More: Periodicity of Valence or Oxidation States of Elements
Understanding ionization energy is also key to explaining metal reactivity and trends in oxidation states.
3. Electronegativity Trend
Electronegativity refers to an atom’s ability to attract and hold electrons when bonded to another atom. This property determines how polar a bond will be.
- Across a Period: Electronegativity increases. Atoms have more protons and stronger nuclear pull, attracting bonding electrons more effectively.
- Down a Group: Electronegativity decreases. Larger atomic radii and increased shielding reduce the nucleus’s pull on bonding electrons.
This trend helps in determining molecule polarity and the type of bond (ionic, polar covalent, non-polar covalent).
4. Metallic and Non-Metallic Character
- Metallic character increases down a group and decreases across a period. Metals tend to lose electrons easily.
- Non-metallic character increases across a period and decreases down a group. Non-metals tend to gain or share electrons.
This is why alkali metals are more reactive at the bottom of Group 1, and why halogens at the top of Group 17 are more reactive than those at the bottom.
5. Valency and Oxidation States
Valency refers to the combining power of an element, often based on its outermost electrons. Across a period, valency typically increases and then decreases as the outer shell fills. In a group, elements share the same valency.
Explore: Periodicity of Valence or Oxidation States
Understanding valency is vital for writing chemical formulas and predicting compound structures.
First 20 Elements and Their Trends
The first 20 elements offer a clear, compact model to study periodic trends. From Hydrogen (H) to Calcium (Ca), we observe several interesting variations in chemical and physical properties.
- Period 1 (H to He): Minimal elements but maximum contrast in properties.
- Period 2 (Li to Ne): Atomic size decreases and electronegativity increases steadily.
- Period 3 (Na to Ar): Similar trends continue, with notable increase in non-metallic character.
- Period 4 beginning (K to Ca): Introduction of d-block (transition) elements after calcium, and sharp increase in metallic nature.
These examples show how periodicity is not just theoretical but practically observed.
See: First 20 Elements of the Periodic Table
Applications of Periodic Trends
Chemical Bonding Predictions
Knowing periodic trends helps chemists and students anticipate bonding behavior. For example:
- Elements with low ionization energy (like Na, K) tend to lose electrons and form cations.
- Elements with high electronegativity (like F, O) tend to gain electrons and form anions.
This understanding makes it easier to predict whether a reaction will result in ionic, covalent, or metallic bonding.
Reactivity Patterns
The reactivity of elements follows a predictable pattern due to periodicity:
- Alkali metals become more reactive down the group.
- Halogens become less reactive as you go down the group.
This helps explain trends in flame colors, reactions with water, and usage in industrial processes.
Real-World and Industrial Applications
Periodic trends influence practical decisions in many fields:
- Batteries: Reactivity trends help select suitable electrodes.
- Catalysts: Transition metals chosen based on oxidation states and ionization energy.
- Medical field: MRI contrast agents and radioactive tracers chosen for specific periodic behaviors.
Understanding these applications gives students a real-world context for abstract chemistry.

FAQs About Periodicity in the Periodic Table
1. What are periodic trends?
Periodic trends are repeating patterns in the physical and chemical properties of elements across the periodic table.
2. Why does atomic radius decrease across a period?
As protons increase in the nucleus, they pull electrons more strongly, making the atom smaller across a period.
3. What is the trend in ionization energy?
It increases across a period due to stronger nuclear attraction and decreases down a group due to increased electron shielding.
4. Why does electronegativity decrease down a group?
Atoms become larger and their outer electrons are less tightly held, reducing their ability to attract bonding electrons.
5. What are the practical uses of periodicity?
It helps in designing chemical reactions, creating new compounds, and understanding reactivity and bonding patterns.
Conclusion
Periodicity in the periodic table isn’t just a classroom concept – it’s a scientific tool that simplifies complex chemistry. It explains why elements behave the way they do, allowing students and researchers to anticipate outcomes and build connections.
Whether you’re memorizing the first 20 elements or exploring trends in ionization energy and electronegativity, periodicity serves as a guide. It connects atomic structure to real-world chemistry, giving you a powerful framework for learning.
Dive deeper into individual trends and elemental behaviors with Deeksha’s trusted learning modules. From atomic structure to valency and oxidation states, enhance your preparation with clarity and confidence.
Table of Contents









Get Social