Dalton’s Atomic Theory, proposed by John Dalton in 1808, marks the beginning of modern chemical science. It provided a logical explanation for the Laws of Chemical Combination and introduced the concept of atoms as the smallest, indivisible particles of matter. This theory bridged experimental observations with theoretical chemistry, forming the backbone of atomic structure understanding used in both school-level and competitive exams such as JEE.
Dalton developed this theory to explain why elements always combine in fixed proportions and how chemical reactions occur through the rearrangement of atoms. His ideas revolutionized chemistry, transforming it from a qualitative to a quantitative science.
Postulates of Dalton’s Atomic Theory
- All matter is made up of tiny, indivisible particles called atoms. These atoms cannot be created, destroyed, or subdivided by ordinary chemical means.
- Atoms of a given element are identical in all respects, including mass and chemical properties.
- Atoms of different elements differ in mass and other properties.
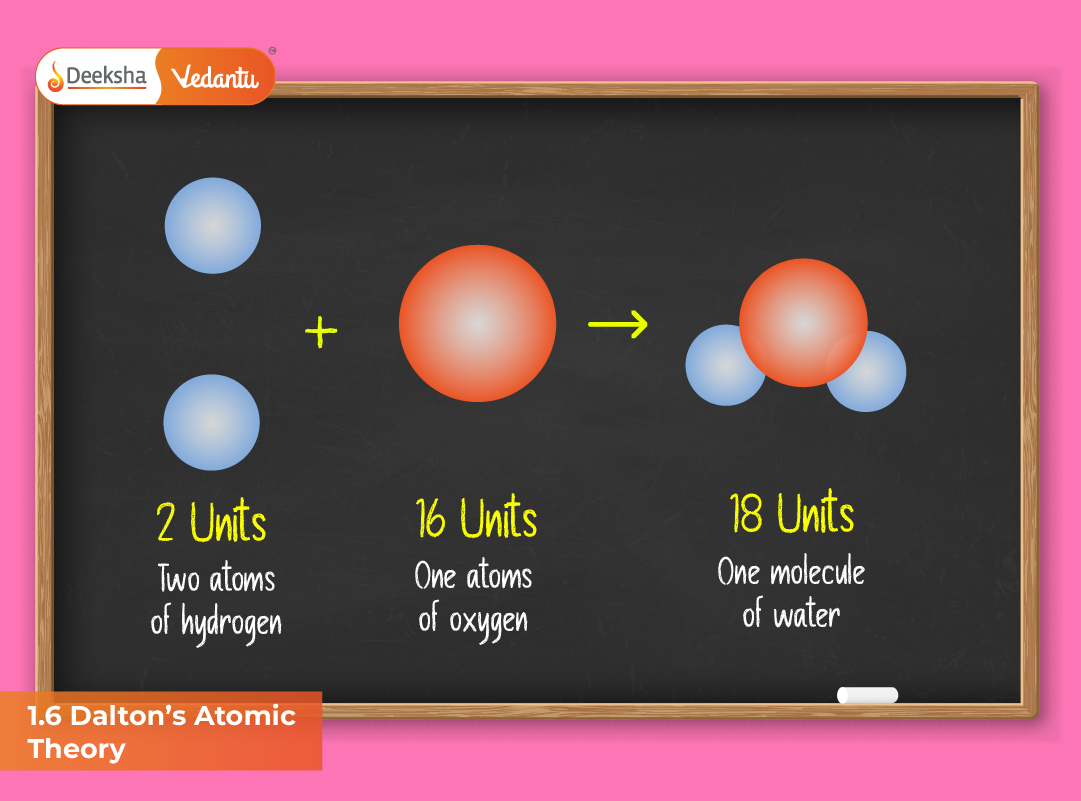
- Compounds are formed when atoms of different elements combine in simple whole-number ratios.
- Chemical reactions involve rearrangement of atoms. No atoms are created or destroyed during a chemical change.
These postulates explained the laws of conservation of mass, definite proportions, and multiple proportions, connecting Dalton’s ideas with previous experimental findings.
Explanation of Dalton’s Postulates
Dalton’s ideas helped scientists interpret chemical reactions on an atomic level:
- Indivisibility of atoms: Atoms were considered the smallest building blocks of matter. Although later discoveries (electrons, protons, neutrons) showed that atoms are divisible, Dalton’s concept was crucial for progress.
- Uniformity within an element: This postulate explained why a given element exhibits consistent chemical behavior. For instance, all oxygen atoms react similarly because they have identical atomic properties.
- Formation of compounds: When hydrogen and oxygen combine to form water, they do so in a fixed ratio of 2:1 by volume, reflecting Dalton’s simple whole-number principle.
- Conservation of mass: Since atoms are neither created nor destroyed, total mass remains constant in reactions.
Limitations of Dalton’s Atomic Theory
- Atoms are not indivisible. The discovery of subatomic particles (electrons, protons, neutrons) proved that atoms have internal structures.
- Isotopes disprove atomic identity. Atoms of the same element can have different masses (e.g., hydrogen-1, hydrogen-2, hydrogen-3), contradicting Dalton’s assumption.
- Isobars exist. Different elements can have the same atomic mass but different properties.
- Dalton’s theory could not explain bonding. It failed to describe how atoms combine to form compounds or the nature of chemical forces.
- Does not address atomic energy. Modern atomic models (Bohr, Schrödinger) consider energy levels and subatomic behavior, absent in Dalton’s time.
Despite these limitations, Dalton’s theory remains a cornerstone because it introduced the atomic concept and quantitative basis for chemical reactions.
Importance of Dalton’s Atomic Theory in Modern Chemistry
Dalton’s ideas established the foundation for:
- Understanding atomic and molecular weights.
- Development of chemical formulas and equations.
- Introduction of mole concept and Avogadro’s number.
- Derivation of atomic models by Thomson, Rutherford, and Bohr.
It is also essential for interpreting the Laws of Chemical Combination — particularly conservation of mass, definite proportions, and multiple proportions.
JEE-Oriented Insights
- Expected Weightage: 1–2 marks in JEE Main; conceptual questions are common.
- Common Topics: Application of laws, atomic structure basics, isotopes, and early atomic models.
- Tip: Remember which postulates correlate with each law — this often appears in assertion-reason questions.
FAQs
Q1. Who proposed Dalton’s Atomic Theory and when?
John Dalton proposed the Atomic Theory in 1808 to explain how elements combine chemically in definite ratios.
Q2. What are the key postulates of Dalton’s Atomic Theory?
The theory states that matter consists of indivisible atoms, identical for each element, combining in simple ratios to form compounds, and conserved during reactions.
Q3. What are the main limitations of Dalton’s Atomic Theory?
It failed to explain isotopes, isobars, subatomic particles, and the nature of chemical bonding.
Q4. How is Dalton’s Atomic Theory related to the Law of Conservation of Mass?
It supports the law by asserting that atoms are neither created nor destroyed during chemical reactions; they only rearrange.
Q5. What is the importance of Dalton’s Atomic Theory in modern chemistry?
It laid the foundation for molecular and atomic understanding, leading to the development of the periodic table and quantum models.
Conclusion
Dalton’s Atomic Theory was revolutionary for its time. It provided the first systematic framework to understand matter’s composition and behavior. While modern discoveries have refined these ideas, Dalton’s theory remains a fundamental stepping stone in the evolution of chemistry. For JEE aspirants, a solid grasp of this topic is essential to comprehend later topics like atomic structure, molecular theory, and stoichiometry.





Get Social