The periodic table is one of the most important tools in the field of chemistry. It serves as a comprehensive map of all known elements and helps us understand their physical and chemical properties. By studying the periodic table, students can learn how elements are related to each other, how they react, and how to predict their behavior in different chemical reactions. Whether you’re just starting out in chemistry or preparing for board exams, mastering the periodic table provides a strong foundation for deeper learning.
In this guide, we will take a closer look at the periodic table from two perspectives: the first 20 elements that are most commonly studied at the school level and the complete list of all 118 elements known to science. We’ll also explain periodic trends, atomic mass, and electronic configurations in a simplified manner. Let’s dive in.
What Is the Periodic Table?
The periodic table is a tabular arrangement of chemical elements, organized based on their increasing atomic number, electron configurations, and recurring chemical properties. Elements are arranged in horizontal rows known as periods and vertical columns called groups or families. This structured layout helps in recognizing patterns and predicting how elements behave chemically.
The concept of the periodic table was first introduced by Dmitri Mendeleev, who arranged elements based on atomic mass. Later, Henry Moseley revised it to the modern version, where elements are organized by atomic number. Each block of the table gives information about an element’s symbol, atomic number, and atomic mass.
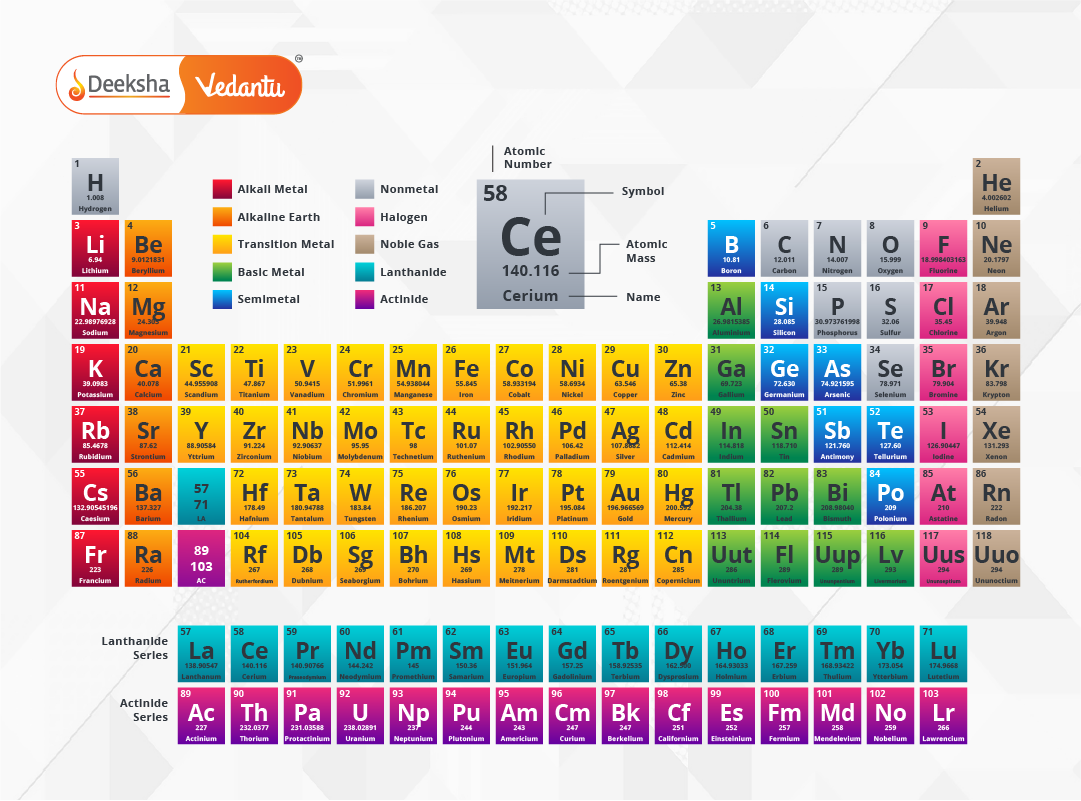
Explore the Modern Periodic Table to understand how elements are scientifically categorized today.
Periodic Table First 20 Elements
The first 20 elements of the periodic table are particularly important for Class 9 and 10 students. These elements include:
| Atomic Number | Element Name | Symbol | Atomic Mass |
| 1 | Hydrogen | H | 1.008 |
| 2 | Helium | He | 4.0026 |
| 3 | Lithium | Li | 6.94 |
| 4 | Beryllium | Be | 9.0122 |
| 5 | Boron | B | 10.81 |
| 6 | Carbon | C | 12.01 |
| 7 | Nitrogen | N | 14.01 |
| 8 | Oxygen | O | 16.00 |
| 9 | Fluorine | F | 19.00 |
| 10 | Neon | Ne | 20.18 |
| 11 | Sodium | Na | 22.99 |
| 12 | Magnesium | Mg | 24.31 |
| 13 | Aluminium | Al | 26.98 |
| 14 | Silicon | Si | 28.09 |
| 15 | Phosphorus | P | 30.97 |
| 16 | Sulphur | S | 32.07 |
| 17 | Chlorine | Cl | 35.45 |
| 18 | Argon | Ar | 39.95 |
| 19 | Potassium | K | 39.10 |
| 20 | Calcium | Ca | 40.08 |

These elements represent a mix of metals, non-metals, and noble gases. They are frequently used in school experiments and theoretical examples. Learning their properties, symbols, and valency gives students an essential base for understanding more advanced topics.
Learn more about the First 20 Elements, their uses in daily life, and key characteristics.
Periodic Table All 118 Elements
As of today, scientists have identified and confirmed 118 chemical elements. These elements include naturally occurring ones and synthetic elements created in laboratories. Each of these elements has a unique atomic number, symbol, and set of physical and chemical properties.
The full periodic table consists of:
- Alkali metals (Group 1)
- Alkaline earth metals (Group 2)
- Transition metals (Groups 3–12)
- Metalloids
- Non-metals
- Halogens (Group 17)
- Noble gases (Group 18)
- Lanthanides and actinides
Having a full view of all 118 elements allows students to explore periodic trends more deeply and understand the scope of chemical behavior.
Visit our reference page on All 118 Elements and Their Symbols to view the complete table.
Periodic Trends Explained
Periodic trends refer to predictable patterns that occur in the periodic table based on an element’s position. These trends help explain why elements behave the way they do.
1. Atomic Radius
- The size of an atom is measured by its atomic radius.
- It decreases across a period (left to right) because the number of protons increases, pulling electrons closer.
- It increases down a group as new shells are added.
2. Ionization Energy
- The energy required to remove an electron from an atom.
- Increases across a period due to stronger nuclear charge.
- Decreases down a group as electrons are further from the nucleus.
3. Electron Affinity
- The amount of energy released when an atom gains an electron.
- Becomes more negative across a period, indicating stronger attraction.
- Becomes less negative down a group.
4. Electronegativity
- A measure of an atom’s ability to attract shared electrons.
- Increases across a period due to stronger nuclear charge.
- Decreases down a group due to increased atomic size.
Understanding these periodic trends gives students insights into element bonding and reactivity patterns.
Atomic Mass and Electronic Configuration
Atomic Mass:
Atomic mass is the weighted average of all the isotopes of an element. It is roughly equal to the number of protons and neutrons in the nucleus.
Electronic Configuration:
This refers to the distribution of electrons in different shells of an atom.
For example:
- Carbon (C): 2, 4
- Oxygen (O): 2, 6
- Sodium (Na): 2, 8, 1
Understanding configuration helps predict how atoms bond. It also determines whether elements donate, accept, or share electrons in chemical reactions.
Review our guide on Atomic Mass of Elements and Electronic Configuration of First 30 Elements for more clarity.
Why the Periodic Table Matters in Chemistry
The periodic table is more than a chart. It’s a roadmap for students and scientists alike. Here’s why it’s crucial:
- Organizes all known elements for easy reference
- Predicts how elements react based on group trends
- Highlights similarities and differences between element families
- Serves as a foundation for learning about bonding, reactions, and molecular structures
From school-level chemistry to advanced research, the periodic table remains the backbone of chemical science.
FAQs
1. What is the periodic table?
The periodic table is a systematic chart that lists all known chemical elements in order of increasing atomic number, showing periodic trends in their properties.
2. Why are the first 20 elements important?
They are commonly encountered in school-level chemistry and cover a broad range of properties and element types, making them ideal for introducing basic concepts.
3. What is a periodic trend?
It is a recurring pattern in element properties across periods or groups in the periodic table, such as changes in atomic radius or electronegativity.
4. How many elements are there in the periodic table?
There are currently 118 confirmed elements, including naturally occurring and man-made elements.
5. What is the use of learning atomic mass and configuration?
They help in understanding an element’s reactivity, bonding potential, and its role in forming chemical compounds.
By mastering the periodic table, students open the door to exploring the world of chemistry with confidence and curiosity.
Conclusion
Understanding the periodic table – from the first 20 elements to all 118 – is foundational for every chemistry learner. It not only organizes the elements we encounter in nature and labs but also reveals deep insights into their behavior, structure, and reactivity. By grasping periodic trends, atomic mass, and electronic configuration, students can better predict chemical reactions, understand element properties, and build a solid base for future studies in science. Whether you’re preparing for school exams or simply curious about the elements, mastering this table equips you with the knowledge to explore the microscopic world with clarity and purpose.
Table of Contents






Get Social