Atomic structure forms the foundation of chemistry and physics. Every substance in the universe, from the air we breathe to the food we eat, is composed of atoms — tiny particles that are the building blocks of matter. Understanding the structure of atoms and how atomic models evolved over time helps us grasp the logic behind chemical reactions, bonding, periodic trends, and more.
In this blog, we’ll explore:
- The early ideas about atomic structure
- Development of atomic models
- Key contributions by Rutherford and Bohr
- Role of subatomic particles
- Electronic configurations and their connection to the periodic table
Whether you’re a Class 9 or Class 11 student or preparing for competitive exams like NEET or JEE, this guide will simplify everything you need to know about atomic structure.
What Is an Atom?
An atom is the smallest unit of matter that retains the properties of an element. It is made up of subatomic particles:
- Protons (p⁺): Positively charged, located in the nucleus
- Neutrons (n⁰): Neutral, also in the nucleus
- Electrons (e⁻): Negatively charged, revolve around the nucleus in orbits or shells
🔗 Learn more about atomic mass of elements
The entire mass of the atom is concentrated in the nucleus, which is incredibly dense, while electrons occupy the surrounding space.
Evolution of Atomic Models
Scientists proposed various atomic models over time to explain atomic structure and chemical behavior. Let’s take a chronological look:
1. Dalton’s Atomic Model (1803)
- Atom is indivisible and indestructible
- All atoms of an element are identical
- Couldn’t explain electrical nature of atoms or subatomic particles
2. Thomson’s Model (1897)
Also known as the Plum Pudding Model:
- Atom is a positively charged sphere with electrons embedded like “plums”
- Explained electrical neutrality but failed to describe electron arrangement
Rutherford’s Atomic Model
In 1911, Ernest Rutherford conducted the famous gold foil experiment. He bombarded thin gold foil with alpha particles and observed that:
- Most particles passed straight through
- Some were deflected at angles
- A few bounced back
Key Conclusions:
- Atom is mostly empty space
- Positive charge and most mass are concentrated in a small nucleus
- Electrons revolve around the nucleus
Limitations:
- Could not explain the stability of atoms
- Failed to explain the line spectra of elements
🔗 Read more: Rutherford’s Model and Its Limitations
Bohr’s Atomic Model
In 1913, Niels Bohr modified Rutherford’s model to explain atomic stability and spectral lines. He proposed:
Bohr’s Postulates:
- Electrons revolve in discrete orbits or energy levels (shells)
- Energy of electron is quantized; it does not lose energy while in an orbit
- Electrons emit/absorb energy only when they jump between levels
Energy Level Notation:
- Shells are labeled as K, L, M, N… or n=1,2,3,4…
Successes:
- Explained hydrogen atom spectra
- Introduced concept of quantized energy
Limitations:
- Failed to explain multi-electron atoms
- Couldn’t account for fine spectral lines and Zeeman effect
🔗 Explore Bohr’s Model in Detail
Subatomic Particles and Their Properties
| Particle | Symbol | Charge | Mass (kg) | Location |
| Proton | p⁺ | +1 | Nucleus | |
| Neutron | n⁰ | 0 | Nucleus | |
| Electron | e⁻ | -1 | Electron Shell |
These particles determine the atom’s mass, charge, and behavior in chemical reactions.
Electronic Configuration
Electronic configuration is the arrangement of electrons in different energy levels or shells.
Rules:
- Aufbau Principle: Fill lowest energy orbitals first
- Pauli Exclusion Principle: Each orbital holds 2 electrons with opposite spins
- Hund’s Rule: Electrons occupy degenerate orbitals singly first
Example: Electronic configuration of:
- Hydrogen (Z=1): 1s¹
- Oxygen (Z=8): 1s² 2s² 2p⁴
🔗 Electronic Configuration of First 30 Elements
Electronic configuration explains:
- Chemical reactivity
- Valency
- Periodic trends
Quantum Numbers (Advanced)
Quantum numbers describe the unique position of an electron in an atom:
- Principal (n): Shell number (n=1,2,3…)
- Azimuthal (l): Subshell (s, p, d, f)
- Magnetic (m): Orientation of orbital
- Spin (s): +½ or -½
Understanding quantum numbers helps visualize how electrons fill orbitals.
Modern Atomic Model
The current understanding combines quantum mechanics and wave-particle duality.
- Electrons don’t move in fixed orbits but in probability clouds (orbitals)
- Introduced by Erwin Schrödinger through wave equation
- Supports Heisenberg’s Uncertainty Principle: Cannot know exact position and momentum of electron simultaneously
This model is essential in explaining chemical bonding and molecular shapes.
Atomic Structure and the Periodic Table
The structure of atom influences the modern periodic table:
- Elements are arranged by atomic number (Z)
- Periods = number of shells
- Groups = number of valence electrons
- Similar configurations → Similar chemical properties
🔗 Learn How Atomic Structure Shapes the Modern Periodic Table
Example:
- Group 1 (Alkali metals): 1 valence electron → Highly reactive
- Group 18 (Noble gases): Full outer shell → Inert
Real-Life Applications of Atomic Models
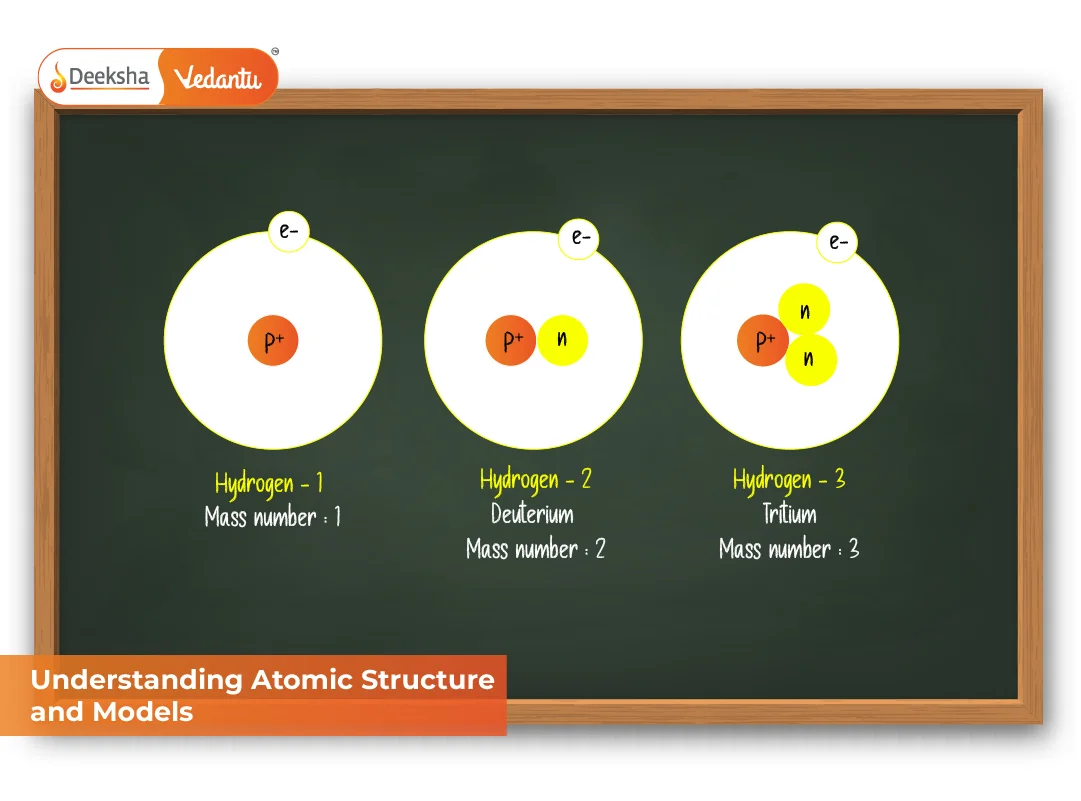
- Nuclear Medicine: Uses isotopes (different neutron count) for diagnosis
- Semiconductors: Silicon’s atomic structure enables computer chips
- Spectroscopy: Bohr’s model explains emission spectra
- Atomic Clocks: Based on electron transitions in atoms
- Quantum Computing: Relies on quantum behavior of subatomic particles
Sample Problem: Bohr Model
Problem: Calculate the energy difference when an electron in hydrogen jumps from n=3 to n=2.
Solution: Use the Bohr energy equation:
Energy released as light = 1.89 eV.
FAQs
Q1. What is the structure of atom?
It consists of a dense nucleus with protons and neutrons, surrounded by electrons arranged in shells.
Q2. What is the Bohr model of atom?
Bohr’s model proposes that electrons revolve in fixed energy levels without radiating energy, and jump levels by absorbing or emitting energy.
Q3. What are subatomic particles?
Particles smaller than atoms — protons, neutrons, and electrons.
Q4. Why did Bohr’s model fail?
It couldn’t explain spectra of multi-electron atoms or certain magnetic effects.
Q5. How are atomic models connected to the periodic table?
Electronic configurations derived from atomic models determine element placement and properties in the periodic table.
Conclusion
Understanding the structure of atoms and the evolution of atomic models is key to mastering chemistry. From Dalton’s simplicity to Bohr’s quantized orbits and modern quantum models, each step has brought us closer to understanding the invisible world that governs everything.
Table of Contents









Get Social