The carbon cycle is one of the most important processes in Earth’s environmental chemistry. It explains how carbon, a fundamental element for life, moves through the air, oceans, soil, plants, and animals. Without this cycle, life as we know it would not exist-and disruptions to it have led to many of the environmental challenges we face today.
In this blog, we’ll explore:
- What the carbon cycle is and how it works
- The role of carbon compounds in the environment
- Carbon’s connection to global warming and pollution
- What environmental chemistry tells us about carbon
- How individuals and industries can reduce their carbon footprint
Let’s begin by understanding the element at the heart of this entire cycle-carbon.
Why Is Carbon So Important?
Carbon is the fourth most abundant element in the universe and essential for life. It forms the backbone of organic molecules such as proteins, carbohydrates, fats, and DNA. Carbon’s unique ability to form stable bonds with many elements makes it a central figure in biological and environmental systems.
Some key forms of carbon in nature include:
- Carbon dioxide (CO₂) – gas released by respiration, combustion, and decay
- Methane (CH₄) – potent greenhouse gas from decomposition and livestock
- Calcium carbonate (CaCO₃) – found in shells, limestone, and coral reefs
- Organic carbon – in all living and decomposing matter
Carbon exists in solid, liquid, and gaseous states. In its solid form (graphite, diamond), it has industrial uses, while in gaseous form (CO₂ and CH₄), it plays critical roles in atmospheric chemistry.
🔗 Explore more about Natural Resources and their chemistry
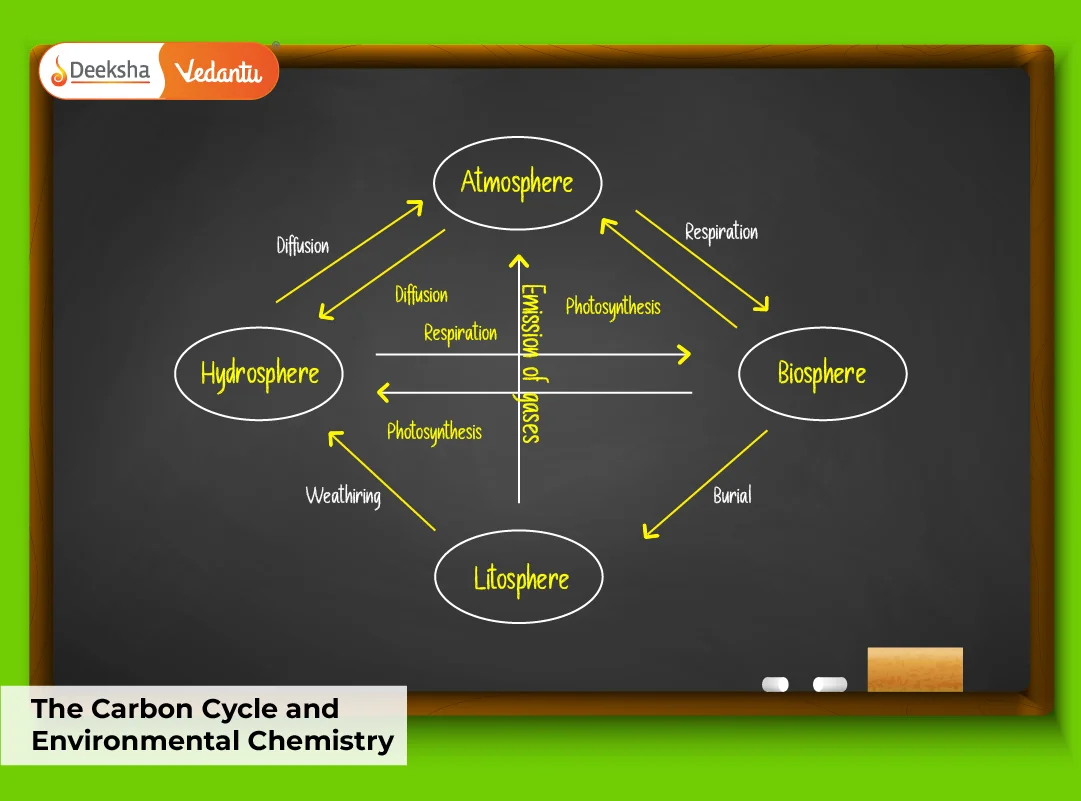
What Is the Carbon Cycle?
The carbon cycle is the natural circulation of carbon through the biosphere, atmosphere, oceans, and geosphere. It includes several processes that add and remove carbon from the environment.
Key Processes in the Carbon Cycle:
| Process | Description |
| Photosynthesis | Plants absorb CO₂ and convert it into glucose using sunlight |
| Respiration | Organisms release CO₂ during the breakdown of food |
| Decomposition | Dead organisms decay, releasing CO₂ and CH₄ into the air and soil |
| Combustion | Burning fossil fuels and wood releases CO₂ |
| Ocean Exchange | CO₂ dissolves in oceans and can be stored or released |
| Sedimentation | Carbon in shells/skeletons becomes limestone over millions of years |
This cycle maintains Earth’s carbon balance, ensuring the planet stays hospitable. Any disturbance in one component, such as increased combustion, can cause serious shifts in climate patterns.
The Slow and Fast Carbon Cycles
- Fast carbon cycle: Movement of carbon through living organisms and the atmosphere (photosynthesis, respiration, etc.)
- Slow carbon cycle: Geological processes like sedimentation, fossil fuel formation, and volcanic eruptions
Human Impact on the Carbon Cycle
Modern industrialization and deforestation have drastically increased carbon emissions. Some major causes include:
- Burning of fossil fuels (coal, oil, gas)
- Cutting down forests (reduces CO₂ absorption)
- Agriculture and livestock (methane release from cattle, rice paddies)
- Waste mismanagement (landfills release methane during organic matter decomposition)
These activities lead to an excess of CO₂ in the atmosphere, which contributes to global warming and climate change.
Rising global temperatures disrupt weather patterns, increase sea levels, and lead to extreme events like wildfires, floods, and prolonged droughts. These are direct consequences of the imbalance in the carbon cycle.
🔗 Learn how carbon imbalance contributes to Global Warming
Environmental Chemistry and Carbon
Environmental chemistry studies how chemical processes affect the Earth’s systems. It helps us understand:
- The behavior of carbon compounds in the environment
- The chemical pathways of pollutants like CO₂ and CH₄
- The formation of acid rain, smog, and greenhouse gases
- Methods to neutralize or reduce harmful emissions
Industrial Emissions and Chemistry
- Factories and power plants release NOx and SO₂ along with CO₂
- These gases form acid rain, which harms aquatic life and soil fertility
- Catalytic converters in vehicles help reduce CO and NOx emissions using redox reactions
Some key insights from environmental chemistry:
- CO₂ + H₂O → H₂CO₃ (carbonic acid): Acidifies oceans and rain
- CH₄ traps 25x more heat than CO₂ over a 100-year period
- Incomplete combustion of fuels creates CO (carbon monoxide), a toxic gas
Green chemistry initiatives focus on reducing harmful emissions at the source rather than treating them after formation.
Carbon Compounds in the Environment
Carbon exists in multiple forms across different ecosystems:
In the Atmosphere:
- Carbon dioxide (CO₂) – greenhouse gas, key contributor to warming
- Methane (CH₄) – from wetlands, cattle, and fossil fuel leaks
- Carbon monoxide (CO) – from vehicle exhaust and industrial burning
In the Biosphere:
- Carbohydrates, lipids, proteins – in plants and animals
- Humus – decaying organic matter in soil
- Plant resins and oils – complex carbon compounds that play a role in plant defense
In the Hydrosphere:
- Dissolved CO₂ – supports marine photosynthesis
- Bicarbonate and carbonate ions – maintain pH in ocean water
- Marine sediments – store carbon long-term in the form of dead plankton and shells
In the Geosphere:
- Fossil fuels – concentrated organic carbon from ancient organisms
- Limestone (CaCO₃) – stores carbon over geological time
- Peat and coal beds – represent partial stages in fossil fuel formation
🔗 Compare with the Nitrogen Cycle here
What Is a Carbon Footprint?
Your carbon footprint is the total amount of greenhouse gases emitted by your actions. It is measured in equivalent tons of CO₂ (CO₂e).
Major Contributors to Personal Carbon Footprint:
- Driving fossil fuel-powered vehicles
- Using electricity from non-renewable sources
- Consuming high-meat diets
- Buying imported goods with high transportation emissions
- Excessive air travel
- Poor waste segregation (organic and inorganic)
Tips to Reduce Carbon Footprint:
- Use public transport, cycle, or walk
- Switch to renewable energy sources
- Reduce, reuse, and recycle
- Eat more plant-based meals
- Conserve water and energy at home
- Practice rainwater harvesting to reduce energy-intensive water usage
- Support afforestation and conservation efforts
🔗 Explore the importance of Rainwater Harvesting
The Role of Forests and Oceans
Forests and oceans act as natural carbon sinks – they absorb more carbon than they release.
Forests:
- Trees absorb CO₂ for photosynthesis
- Forest litter stores organic carbon in soil
- Deforestation and forest fires release stored carbon into the atmosphere
Oceans:
- Act as the largest carbon sink
- Phytoplankton absorb CO₂ during photosynthesis
- Cold polar waters absorb more CO₂ than warmer tropical waters
- Ocean acidification reduces the ability of marine organisms to build shells
However, rising CO₂ levels are causing ocean acidification and coral bleaching – disrupting marine ecosystems.
Ozone Depletion and Carbon Compounds
While ozone layer depletion is caused mainly by CFCs (chlorofluorocarbons), many of these are also potent greenhouse gases.
- CFCs used in refrigerators and aerosols contribute to ozone holes
- They stay in the atmosphere for decades, enhancing both climate change and UV radiation exposure
Ozone depletion and climate change are interlinked. As greenhouse gases rise, they influence stratospheric temperatures, which in turn affect ozone reactions.
🔗 Read more on Ozone Layer Depletion
Sample Problem: Carbon Dioxide Emissions
Problem: A car travels 100 km and emits 180g of CO₂ per km. What is the total emission?
Solution:
This gives a clear picture of how everyday activities contribute to climate change.
You can calculate your household or transportation-related carbon emissions using online carbon footprint calculators.
FAQs
Q1. What is the carbon cycle in simple terms?
It’s the process by which carbon moves between the atmosphere, organisms, oceans, and Earth’s crust.
Q2. How do humans affect the carbon cycle?
Through burning fossil fuels, deforestation, and industrialization – all of which add excess CO₂.
Q3. What is environmental chemistry?
A branch of chemistry that studies how natural and man-made chemicals interact with the environment.
Q4. Why is methane worse than carbon dioxide?
Methane is a more powerful greenhouse gas and traps much more heat in the short term.
Q5. How can I reduce my carbon footprint?
Use clean energy, drive less, recycle, and support sustainable practices.
Q6. What is ocean acidification?
It’s the lowering of ocean pH due to increased CO₂ absorption, affecting marine life and coral reefs.
Q7. Are electric vehicles truly carbon neutral?
They reduce tailpipe emissions, but overall neutrality depends on the source of electricity and battery production.
Conclusion
Understanding the carbon cycle and its chemical context is crucial to protecting our environment. As students, citizens, and future leaders, recognizing how our actions impact the carbon balance helps us build a more sustainable world.
Table of Contents









Get Social