Introduction to Redox Reactions
Redox reactions, short for reduction-oxidation reactions, are fundamental chemical processes where the oxidation states of atoms are changed. This guide will provide a detailed understanding of redox reactions, including their types, mechanisms, and applications in various fields.
What are Redox Reactions?
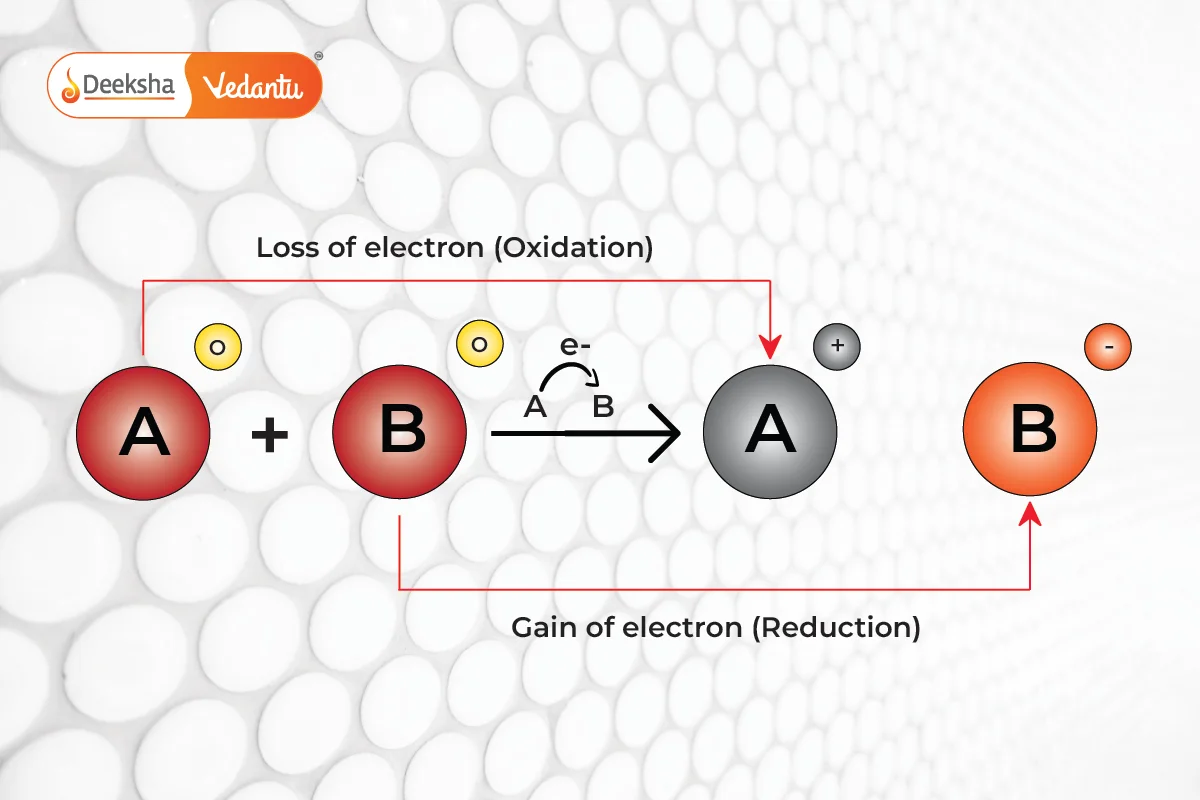
Redox reactions involve the transfer of electrons between two substances. These reactions are composed of two parts: oxidation, where a reactant loses electrons, and reduction, where a reactant gains electrons. The reactant that donates electrons is known as the reducing agent, while the one that accepts electrons is called the oxidizing agent.
Illustration and Mechanism
Consider a scenario where reactant A loses an electron and is oxidized, while reactant B gains this electron and is reduced. This electron shift results in a change in the oxidation states of the reactants, indicating a redox reaction.
Types of Redox Reactions
1. Decomposition Reactions:
This kind of reaction involves the breakdown of a compound into different compounds. Examples of these types of reactions are
All the above reactions result in the breakdown of smaller chemical compounds in the form of
But, there is a special case that confirms that all the decomposition reactions are not redox reactions. These involve the breakdown of a molecule into two or more smaller molecules. For example, the decomposition of water into hydrogen and oxygen.
2. Combination Reactions:
These reactions are the opposite of decomposition reactions and hence, involve the combination of two compounds to form a single compound in the form of . For example,
Opposite to decomposition, these reactions involve the combination of two or more reactants to form one product. An example is the formation of water from hydrogen and oxygen.
3. Displacement Reactions:
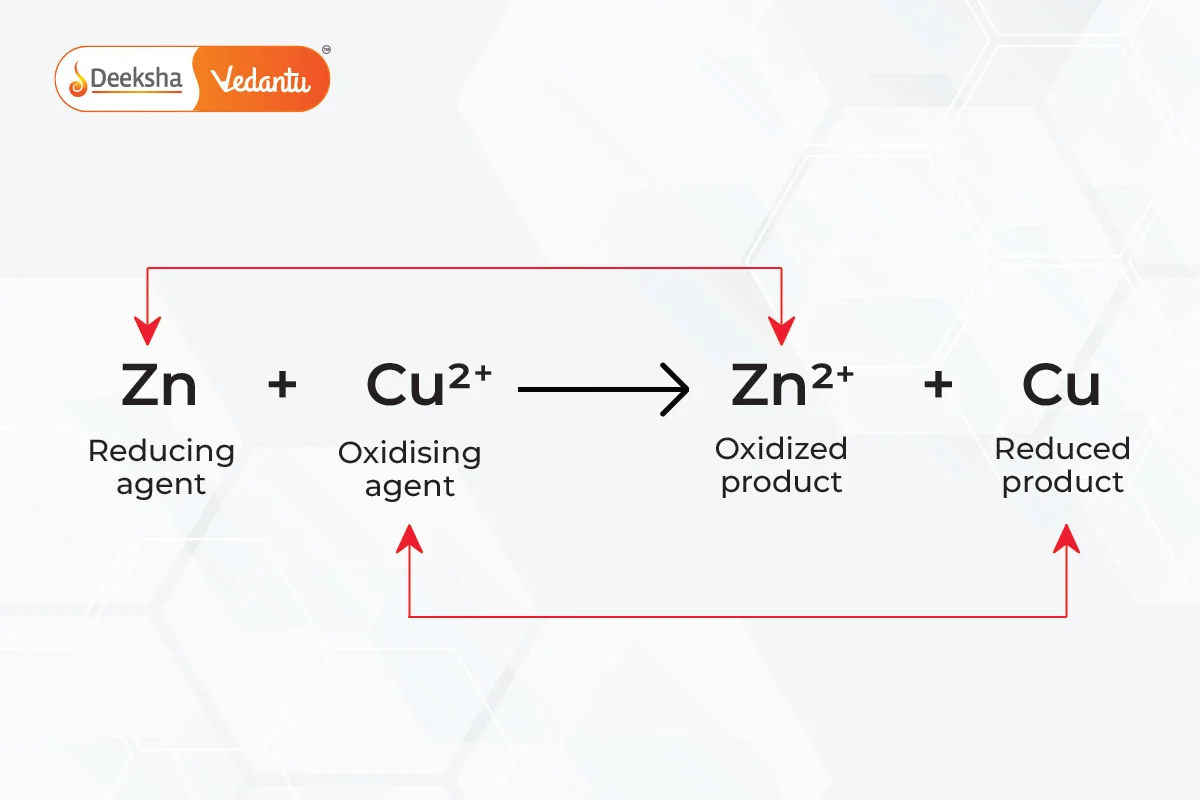
These involve the replacement of an element in a compound by a more reactive element. Examples include the displacement of copper in copper sulfate solution by zinc.
4. Disproportionation Reactions:
These are special types of redox reactions where a single element undergoes both oxidation and reduction. An example is the reaction where hydrogen peroxide acts both as an oxidizing and a reducing agent.
Balancing Redox Reactions
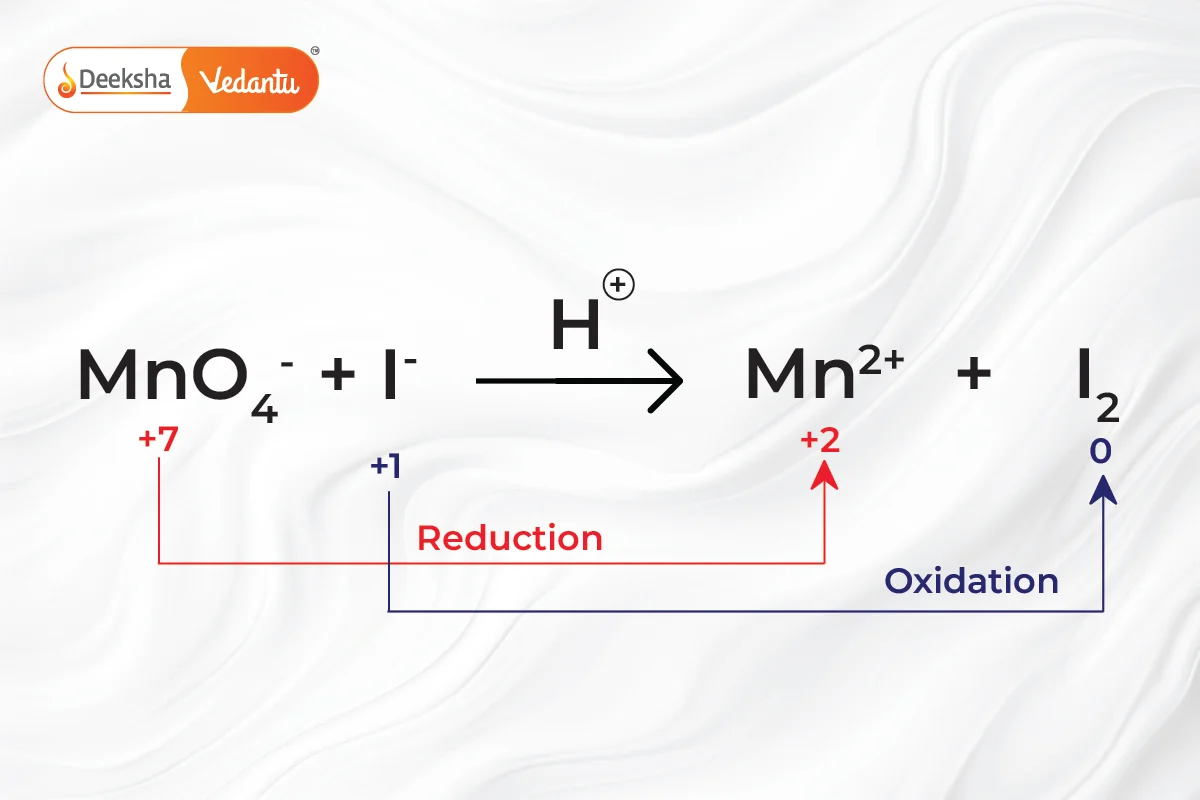
Balancing redox reactions is crucial for the correct representation of chemical changes. The process involves separating the reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is balanced individually for mass and charge.
Applications of Redox Reactions
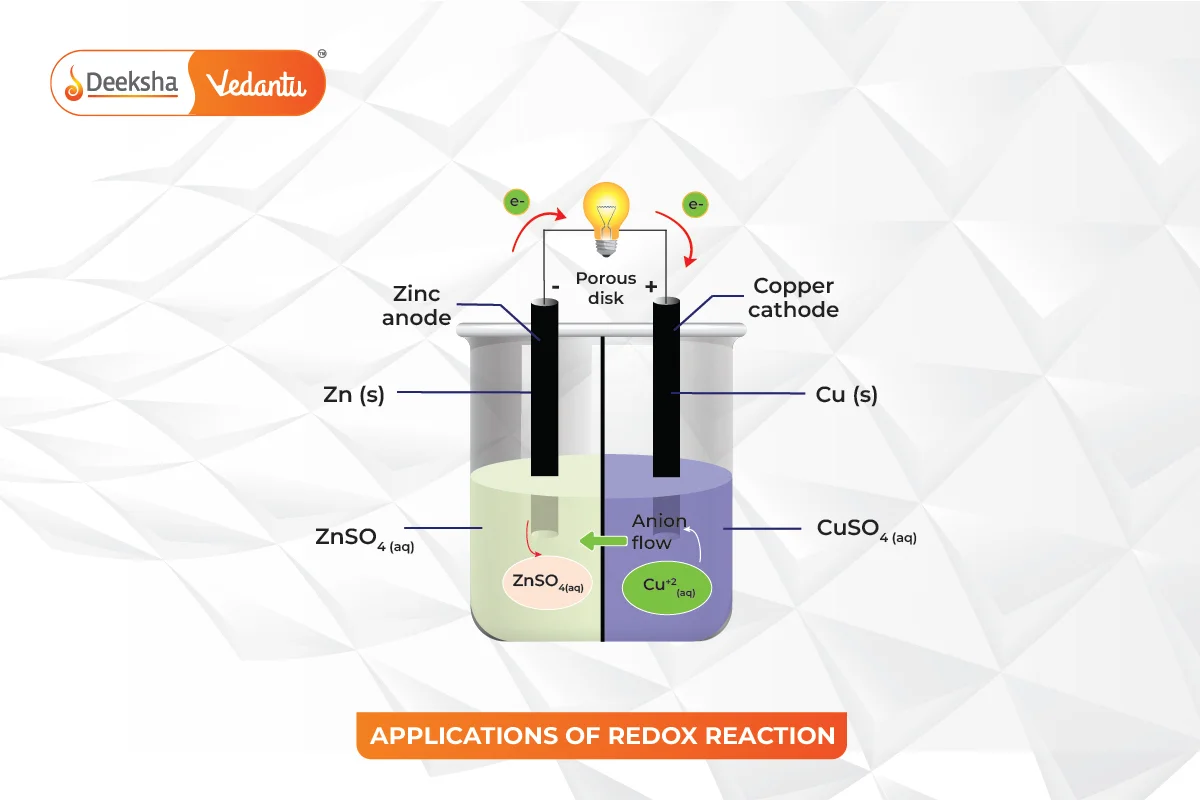
- Electrochemistry:
- Redox reactions are central to the functioning of batteries where chemical energy is converted into electrical energy.
- Industrial Processes:
- Many industrial processes such as the production of chlorine and caustic soda rely on redox reactions.
- Biological Systems:
- Photosynthesis, respiration, and other metabolic processes are examples of redox reactions occurring in biological systems
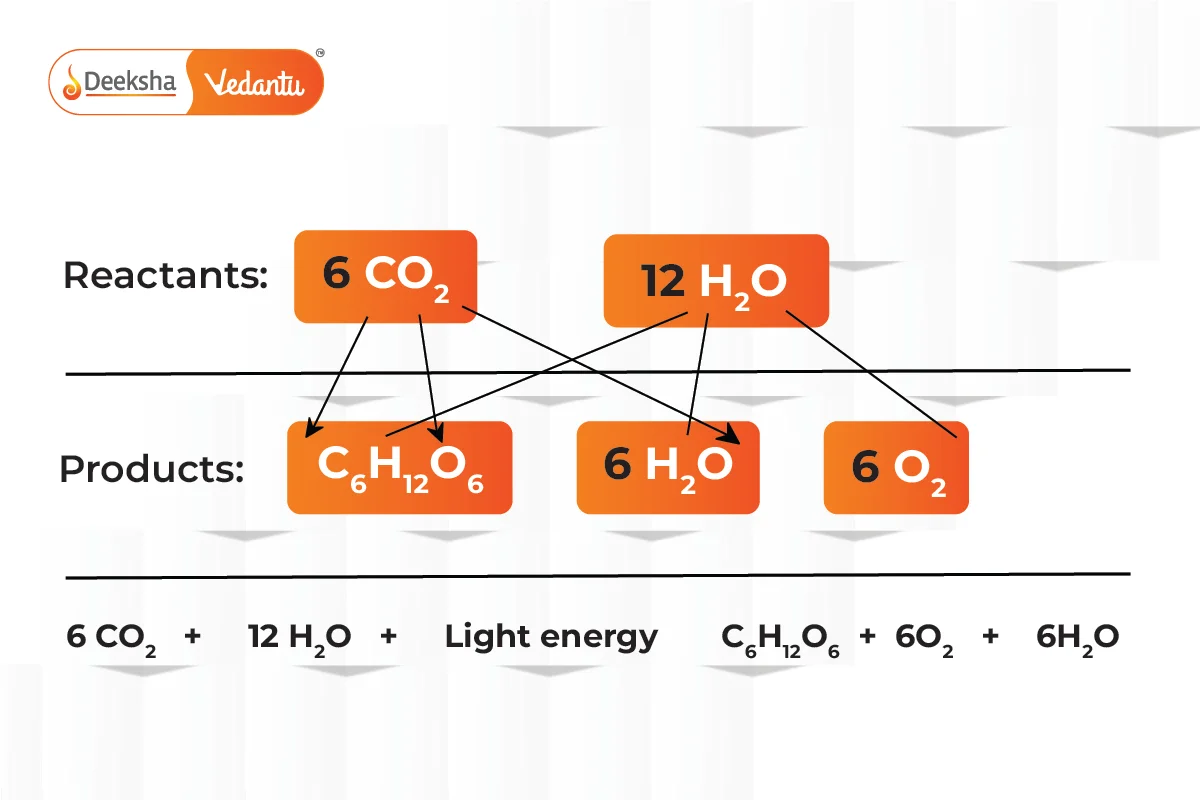
- Combustion and Corrosion:
- Combustion, a rapid form of oxidation, and corrosion processes are also governed by redox reactions.
FAQs
Redox reactions are used in processes like water purification and waste treatment to remove contaminants and toxins.
Balancing ensures that the law of conservation of mass is obeyed, and it allows for the quantitative analysis of the reaction.
Yes, substances like hydrogen peroxide can act as both depending on the chemical environment and the reacting species.
The oxidizing agent gains electrons and is reduced, while the reducing agent loses electrons and is oxidized.
A redox reaction involves the transfer of electrons between two substances, resulting in changes in their oxidation states.





Get Social