What are the First 20 Elements of the Periodic Table?
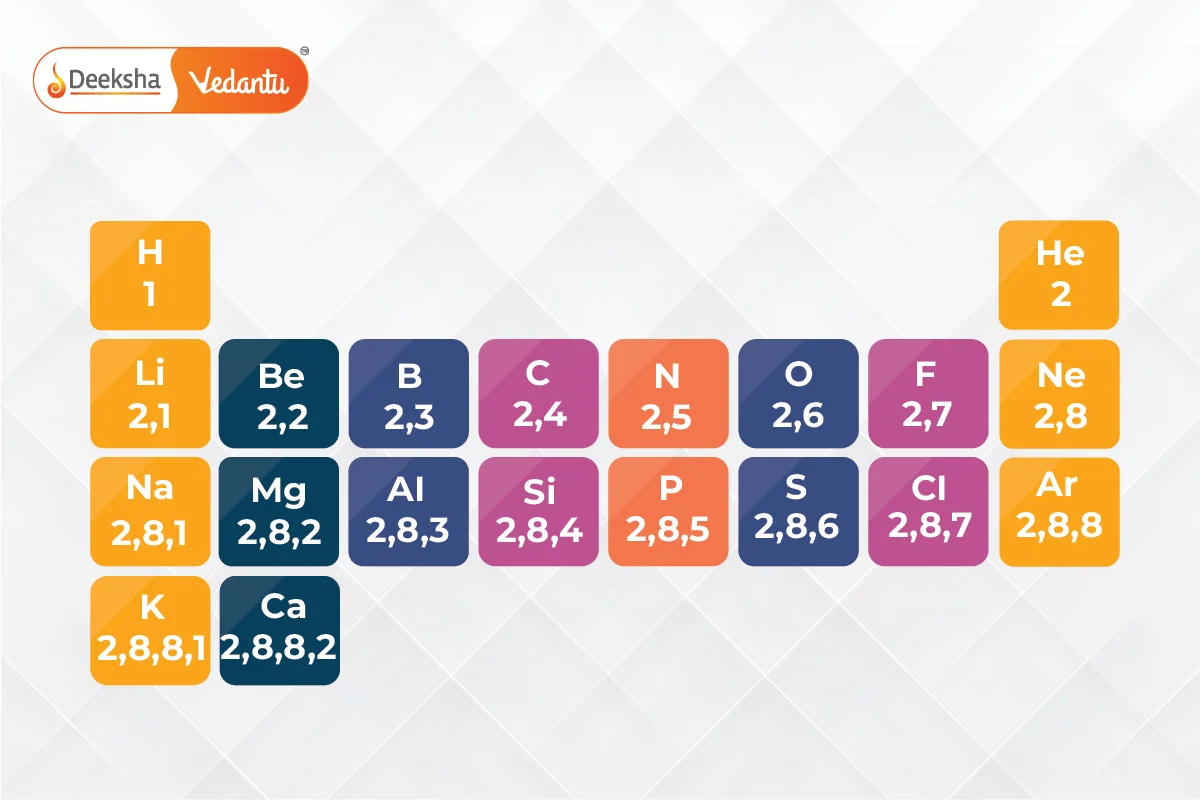
The first 20 elements of the periodic table, with atomic numbers 1 to 20, are:
| Element | Atomic Number | Element Symbol |
| Hydrogen | 1 | H |
| Helium | 2 | He |
| Lithium | 3 | Li |
| Beryllium | 4 | Be |
| Boron | 5 | B |
| Carbon | 6 | C |
| Nitrogen | 7 | N |
| Oxygen | 8 | O |
| Fluorine | 9 | F |
| Neon | 10 | Ne |
| Sodium | 11 | Na |
| Magnesium | 12 | Mg |
| Aluminium | 13 | Al |
| Silicon | 14 | Si |
| Phosphorus | 15 | P |
| Sulphur | 16 | S |
| Chlorine | 17 | Cl |
| Argon | 18 | Ar |
| Potassium | 19 | K |
| Calcium | 20 | Ca |
Importance of The Atomic Number of An Element in A Periodic Table
Atomic Number: The atomic number indicates the number of protons in an atom’s nucleus.
- Protons: Positively charged particles within the nucleus.
- Neutrons: Neutrally charged particles in the nucleus, slightly heavier than protons.
- Electrons: Negatively charged particles orbiting the nucleus, with a mass nearly 1/1836 of a proton.
The atomic number provides:
- The number of protons in the nucleus.
- The number of electrons in a neutral atom.
- The overall charge of the nucleus.
For instance, oxygen, with an atomic number of 8, has 8 protons and 8 electrons in its neutral state. Sodium, with an atomic number of 11, has 11 protons and 11 electrons.
How are the First 20 Elements of the Periodic Table Useful for Us?
- Oxygen (O): Essential for respiration, allowing energy production in most living organisms. It is generated during photosynthesis.
- Carbon (C): Makes up 18% of the human body and is found in proteins, sugars, and other essential compounds. It is also a key component of fossil fuels.
- Aluminum (Al): Used in making products like utensils, airplane parts, and window frames due to its malleability and softness.
- Silicon (Si): A semiconductor crucial for computer chips.
- Phosphorus (P): Used in military applications and as part of ATP, the energy currency of the body.
- Calcium (Ca): Essential for bone strength.
Noble Gases in the First 20 Elements of the Periodic Table
Noble gases, also known as inert gases, are located in group 18 of the periodic table. They are the least reactive elements.
Noble Gases in the First 20 Elements:
- Helium (He): Atomic number 2.
- Neon (Ne): Atomic number 10.
- Atomic number 18.
FAQs
The periodic table arranges elements in order of increasing atomic number, and elements with similar properties are grouped together. This arrangement helps predict an element’s reactivity, state of matter, and other chemical properties based on its position.
- Gases: Hydrogen (H), Helium (He), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne), Argon (Ar).
- Liquids: None.
- Solids: Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Sodium (Na), Magnesium (Mg), Aluminum (Al), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (Cl), Potassium (K), Calcium (Ca).
- Hydrogen (H): Fuel, hydrogenation processes.
- Helium (He): Balloons, cooling superconducting magnets.
- Carbon (C): Organic compounds, fuels.
- Oxygen (O): Breathing, combustion.
- Sodium (Na): Table salt (NaCl), street lights.
- Calcium (Ca): Bones, teeth, cement.
Noble gases are inert, non-reactive gases located in group 18 of the periodic table. Among the first 20 elements, the noble gases are Helium (He), Neon (Ne), and Argon (Ar).
The electronic configuration is determined by the number of electrons, which is equal to the atomic number. Electrons fill energy levels (shells) around the nucleus in a specific order, following the Aufbau principle, Hund’s rule, and the Pauli exclusion principle.
These elements are fundamental in chemistry and biology. They include essential elements for life, such as Oxygen (O) for respiration, Carbon (C) for organic compounds, and Calcium (Ca) for bones.
Element symbols are usually derived from their English names, often using the first one or two letters (e.g., H for Hydrogen, He for Helium). Some symbols are derived from Latin names (e.g., Au from Aurum for Gold, Fe from Ferrum for Iron).
The atomic number represents the number of protons in an atom’s nucleus. It determines the element’s identity and its position in the periodic table. The atomic number also equals the number of electrons in a neutral atom, influencing the element’s chemical properties.
The first 20 elements are Hydrogen (H), Helium (He), Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne), Sodium (Na), Magnesium (Mg), Aluminum (Al), Silicon (Si), Phosphorus (P), Sulfur (S), Chlorine (Cl), Argon (Ar), Potassium (K), and Calcium (Ca).






Get Social